
The relative acidic strengths of benzoic acid, o-toluic acid and p-toluic acid in decreasing order can be given as:
(A) p-toluic acid > o-toluic acid > benzoic acid
(B) o-toluic acid > p-toluic acid > benzoic acid
(C) p-toluic acid > benzoic acid > o-toluic acid
(D) o-toluic acid > benzoic acid > p-toluic acid
Answer
576k+ views
Hint: To understand this question we should know the structure of benzoic acid and toluic acid in the first place. We can account for the relative acid strength of benzoic acid, o-toluic acid and p-toluic acid in means of electronic or physical effect on atoms and stability of carboxylate anion.
Complete answer:
Firstly let's understand represent the structure of benzoic acid, o-toluic acid and p-toluic acid:
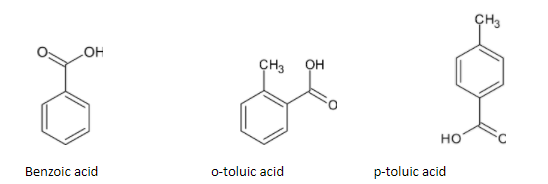
In case of two dissimilar atoms, the more electronegative atom will attract the least electronegative towards themselves that is a displacement takes place. Due to this displacement a partial negative charge on the more electronegative atom and partial positive charge on the least electronegative atom. The induction of polarity in a covalent bond is known as inductive effect. The arrow heading pointing towards the more electronegative atom or group. In a C-X molecule, if C is electronegative than X. This represents +I effect.
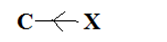
In a C-X molecule, if X is more electronegative than C. This represent -I effect
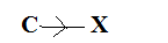
As -I effect increases then acidic character also increases. The steric hindrance also accounts for acidic strength. In aromatic carboxylic acids, having a double bond are less electron donating compared to the saturated carbon towards COOH group. This is because the COOH group is attached to the $s{{p}^{2}}$ hybridized carbon in case of saturated carbon. The relative acid strength of benzoic acid is more but lesser than o-toluic acid. Toluic acid has a methyl group which is an electron withdrawing group. The presence of electron withdrawing groups into the benzene nucleus of aromatic carboxylic acid resulting in an increase in acidic strength. o-toluic acid has higher acidic strength than benzoic acid. As we can see in the above mentioned structure of o-toluic acid. The two larger groups are nearer to each other due to which steric hindrance effect becomes effective. It also undergoes ortho effect. This increases the acidic character. p-toluic acid has the least acidic strength. In the structure of p-toluic acid, we can observe $-C{{H}_{3}}$ and -COOH are located on opposite sides. The $-C{{H}_{3}}$ shows +I effect and -COOH shows -I effect. The electron withdrawing group increases the negative charge on carboxylate anion and destabilizes it . This decreases the acidic nature of p-toluic acid. Thus, the acidic strength trend will be:
o-toluic acid > benzoic acid > p-toluic acid
Hence, option D is the correct answer.
Note: The acidic strength of m-toluic acid is more than p-toluic acid but less than benzoic acid, actually o-toluic acid also should have less acidic strength than benzoic acid due to presence of the electron withdrawing group. But due to ortho effect it's acidic strength is higher than benzoic acid.
Complete answer:
Firstly let's understand represent the structure of benzoic acid, o-toluic acid and p-toluic acid:

In case of two dissimilar atoms, the more electronegative atom will attract the least electronegative towards themselves that is a displacement takes place. Due to this displacement a partial negative charge on the more electronegative atom and partial positive charge on the least electronegative atom. The induction of polarity in a covalent bond is known as inductive effect. The arrow heading pointing towards the more electronegative atom or group. In a C-X molecule, if C is electronegative than X. This represents +I effect.
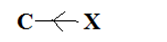
In a C-X molecule, if X is more electronegative than C. This represent -I effect
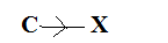
As -I effect increases then acidic character also increases. The steric hindrance also accounts for acidic strength. In aromatic carboxylic acids, having a double bond are less electron donating compared to the saturated carbon towards COOH group. This is because the COOH group is attached to the $s{{p}^{2}}$ hybridized carbon in case of saturated carbon. The relative acid strength of benzoic acid is more but lesser than o-toluic acid. Toluic acid has a methyl group which is an electron withdrawing group. The presence of electron withdrawing groups into the benzene nucleus of aromatic carboxylic acid resulting in an increase in acidic strength. o-toluic acid has higher acidic strength than benzoic acid. As we can see in the above mentioned structure of o-toluic acid. The two larger groups are nearer to each other due to which steric hindrance effect becomes effective. It also undergoes ortho effect. This increases the acidic character. p-toluic acid has the least acidic strength. In the structure of p-toluic acid, we can observe $-C{{H}_{3}}$ and -COOH are located on opposite sides. The $-C{{H}_{3}}$ shows +I effect and -COOH shows -I effect. The electron withdrawing group increases the negative charge on carboxylate anion and destabilizes it . This decreases the acidic nature of p-toluic acid. Thus, the acidic strength trend will be:
o-toluic acid > benzoic acid > p-toluic acid
Hence, option D is the correct answer.
Note: The acidic strength of m-toluic acid is more than p-toluic acid but less than benzoic acid, actually o-toluic acid also should have less acidic strength than benzoic acid due to presence of the electron withdrawing group. But due to ortho effect it's acidic strength is higher than benzoic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




