
The secondary alkyl halide is called as secondary because:
a.) The functional group is attached to primary carbon atom
b.) The functional group is attached to secondary carbon atom
c.) The functional group is attached to tertiary carbon atom
d.) The functional group is attached to secondary non-carbon atom
Answer
601.5k+ views
Hint: Alkyl halide is a compound in which hydrogen is replaced by a functional group, i.e. halogens (fluorine, chlorine, bromine or iodine). ‘Halide’ is another name for ‘halogens’. The compounds containing one, two or more (three, four…) halogens are called mono, di, poly-halogen compounds respectively.
Complete step by step answer:
Alkyl halides are also called haloalkanes. Alkyl halides are those compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). The general formula of alkyl halides is - \[{{C}_{n}}{{H}_{2n+1}}X\].
Or, in simpler words, alkyl halides are those compounds in which a halogen atom is bonded to an alkyl group. They are classified as primary, secondary, tertiary according to the nature of carbon to which the halogen is attached. It can be represented as –R’’’
(R – Alkyl group; X – halogen)
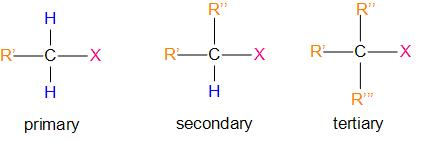 Therefore, the answer is – option (b) – The secondary alkyl halide is called secondary because the functional group is attached to the secondary carbon atom.
Therefore, the answer is – option (b) – The secondary alkyl halide is called secondary because the functional group is attached to the secondary carbon atom.
Additional Information:
Alkyl halides are \[s{{p}^{3}}\]hybridized compounds. Other than this, allylic halides and benzylic halides are also \[s{{p}^{3}}\] hybridized compounds.
Note: The compounds which contain \[s{{p}^{2}}\] C-X bond are divided into two categories –
Vinylic halides
In these compounds, halogen is bonded to \[s{{p}^{2}}\] hybridized carbon atom of C-C double bond.
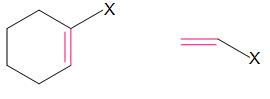 Aryl halides
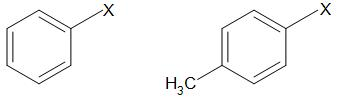
Aryl halides
In these compounds, halogen is bonded to \[s{{p}^{2}}\] hybridized carbon atom of aromatic ring.
Complete step by step answer:
Alkyl halides are also called haloalkanes. Alkyl halides are those compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). The general formula of alkyl halides is - \[{{C}_{n}}{{H}_{2n+1}}X\].
Or, in simpler words, alkyl halides are those compounds in which a halogen atom is bonded to an alkyl group. They are classified as primary, secondary, tertiary according to the nature of carbon to which the halogen is attached. It can be represented as –R’’’
(R – Alkyl group; X – halogen)

Additional Information:
Alkyl halides are \[s{{p}^{3}}\]hybridized compounds. Other than this, allylic halides and benzylic halides are also \[s{{p}^{3}}\] hybridized compounds.
Note: The compounds which contain \[s{{p}^{2}}\] C-X bond are divided into two categories –
Vinylic halides
In these compounds, halogen is bonded to \[s{{p}^{2}}\] hybridized carbon atom of C-C double bond.
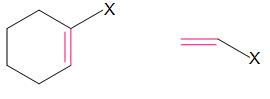
In these compounds, halogen is bonded to \[s{{p}^{2}}\] hybridized carbon atom of aromatic ring.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




