
The shape of a molecule can be determined from its Lewis electron-dot diagram.
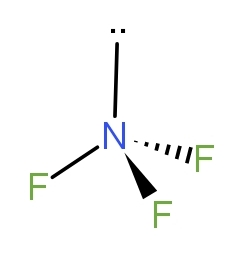
Nitrogen trifluoride ($N{F_3}$) is shown in the diagram below. Which of the following choices has correctly identified both the hybridisation of the central atom and the molecular geometry ?
a.) $s{p^2}$ trigonal planar
b.) $s{p^3}$ pyramidal
c.) sp pyramidal
d.) None of these

Answer
573.3k+ views
Hint: The nitrogen trifluoride is a similar ammonia molecule. It has even hybridisation and shape of the molecule like ammonia molecule. The difference comes just in the bond length because in ammonia N-H bonds are present and in nitrogen trifluoride, N-F bonds are present.
Complete answer:
From the above figure given to us in the question, we can see that the molecule has tetrahedral geometry. As, the Nitrogen has one lone pair and lone pairs are not taken in the shape of the molecule. So, the molecule has a pyramidal shape. So, if we see the hybridisation. We can calculate that as -
Number of hybrid orbitals = Number of sigma bonds + Number of lone pairs
Number of hybrid orbitals = 3 + 1
Number of hybrid orbitals = 4
So, the hybridisation of the molecule is $s{p^3}$.
Thus, the correct answer is option b.).
Note: It must be noted that Nitrogen trifluoride is colourless, non-flammable gas. It has a musty odour. It is a greenhouse gas. It has a wide number of applications. It is used in plasma etching of silicon wafers. It is used to clean PECVD chambers for the high volume production of LCDs. It is used in the hydrogen fluoride and deuterium fluoride lasers which are the type of chemical lasers.
Complete answer:
From the above figure given to us in the question, we can see that the molecule has tetrahedral geometry. As, the Nitrogen has one lone pair and lone pairs are not taken in the shape of the molecule. So, the molecule has a pyramidal shape. So, if we see the hybridisation. We can calculate that as -
Number of hybrid orbitals = Number of sigma bonds + Number of lone pairs
Number of hybrid orbitals = 3 + 1
Number of hybrid orbitals = 4
So, the hybridisation of the molecule is $s{p^3}$.
Thus, the correct answer is option b.).
Note: It must be noted that Nitrogen trifluoride is colourless, non-flammable gas. It has a musty odour. It is a greenhouse gas. It has a wide number of applications. It is used in plasma etching of silicon wafers. It is used to clean PECVD chambers for the high volume production of LCDs. It is used in the hydrogen fluoride and deuterium fluoride lasers which are the type of chemical lasers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




