
The shape of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\]ion is
A.Hexagonal
B.Pyramidal
C.Octahedral
D.Octagonal
Answer
496.8k+ views
Hint: To find the shape we need to find the hybridization. To find hybridization, first draw the Lewis structure to get a rough idea about the structure of the molecule and bonding pattern. Use the valence concept to arrive at this structure. Concentrate on the electron pairs and other atoms linked directly to the concerned atom. Using the Lewis structure, calculate the number of sigma (\[\sigma \]) bonds and the number of lone pairs. Then calculate the steric number. Steric number is the sum of the number of \[\sigma \]-bonds and the number of lone pairs. Now, based on the steric number, it is possible to get the type of hybridization of the atom. Consult the following table.
Complete answer:
Number of \[\sigma \]-bonds formed by the atom in a compound is equal to the number of other atoms with which it is directly linked to.
The number of lone pairs on a given atom can be calculated by using the following formula.
Number of lone pairs\[ = \dfrac{{v - b - c}}{2}\]
Where \[v\] is number of valence electrons in the concerned atom in free state (i.e., before bond formation), \[b\]is number of bonds (including both \[\sigma \]and \[\pi \]bonds) formed by concerned atom and \[c\]is charge on the atom.
The Lewis structure of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\]
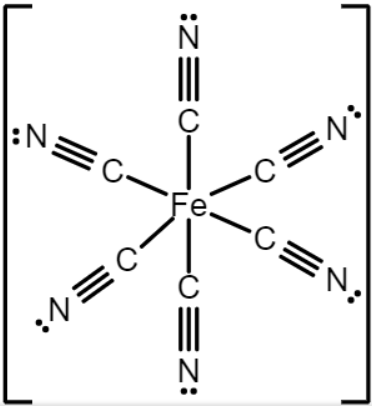
From the above structure, the central atom of iron (\[F{e^{ + 2}}\]) is having \[6\] sigma bonds and no lone pair. Which implies that the steric number of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\]is equals to \[6\] and then the hybridization is \[s{p^3}d\].
Hence the geometry\shape of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\] is octahedral.
Thus, the correct option is (C) Octahedral.
Note:
Planarity or nonplanarity is determined by shape/geometry which is further controlled by hybridisation of the central atom in the compound so by knowing the hybridisation we can tell whether a compound is planar or not. Hence, the molecule will not be planar if there is an \[s{p^3}\] hybridized carbon (or nitrogen) atom or two \[s{p^2}\] hybridized atoms of carbon (or nitrogen) which are separated by an even number of double bonds and no single bonds. Otherwise, its structure allows it to be planar. The polarity of a molecule is basically the measure of its dipole moment. If the dipole is a considerable number (not equal to zero), the molecule is said to be polar.
| Steric number | hybridization | Structure |
| \[2\] | \[sp\] | Linear |
| \[3\] | \[s{p^2}\] | Trigonal planar |
| \[4\] | \[s{p^3}\] | tetrahedral |
| \[5\] | \[s{p^3}d\] | Trigonal bipyramidal |
| \[6\] | \[s{p^3}{d^2}\] | octahedral |
| \[7\] | \[s{p^3}{d^3}\] | Pentagonal bipyramidal |
Complete answer:
Number of \[\sigma \]-bonds formed by the atom in a compound is equal to the number of other atoms with which it is directly linked to.
The number of lone pairs on a given atom can be calculated by using the following formula.
Number of lone pairs\[ = \dfrac{{v - b - c}}{2}\]
Where \[v\] is number of valence electrons in the concerned atom in free state (i.e., before bond formation), \[b\]is number of bonds (including both \[\sigma \]and \[\pi \]bonds) formed by concerned atom and \[c\]is charge on the atom.
The Lewis structure of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\]

From the above structure, the central atom of iron (\[F{e^{ + 2}}\]) is having \[6\] sigma bonds and no lone pair. Which implies that the steric number of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\]is equals to \[6\] and then the hybridization is \[s{p^3}d\].
Hence the geometry\shape of \[{\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}\] is octahedral.
Thus, the correct option is (C) Octahedral.
Note:
Planarity or nonplanarity is determined by shape/geometry which is further controlled by hybridisation of the central atom in the compound so by knowing the hybridisation we can tell whether a compound is planar or not. Hence, the molecule will not be planar if there is an \[s{p^3}\] hybridized carbon (or nitrogen) atom or two \[s{p^2}\] hybridized atoms of carbon (or nitrogen) which are separated by an even number of double bonds and no single bonds. Otherwise, its structure allows it to be planar. The polarity of a molecule is basically the measure of its dipole moment. If the dipole is a considerable number (not equal to zero), the molecule is said to be polar.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




