
The shortest distance between two tetrahedral voids is:
(A) $ \text{a}\sqrt{\text{3}} $
(B) $ \dfrac{\text{a}\sqrt{\text{3}}}{2} $
(C) $ \dfrac{\text{a}}{2} $
(D) $ \dfrac{\text{a}\sqrt{\text{3}}}{4} $
Answer
552k+ views
Hint: The voids in the crystals can be defined as the region of empty space between the molecules of the crystals and this empty space is the region which lies outside the normal van der Waals surface area of molecules in the unit cells and within which no nuclei of the atoms exists.
Complete step by step solution:
To answer this question, let us take the example of the Face Centred Cubic Crystal system. There are a total 8 tetrahedral voids in a Face Centred Cubic lattice. Now if we divide the Face Centred Cubic lattice into 8 smaller cubes then each cube will have one tetrahedral void at the centre of the cube at the body centre of the small cubes. Now if we consider the length of the side of the big Face Centred Cube be a, then on breaking into smaller cubes the edge length of each cube will be $ \dfrac{\text{a}}{2} $ .
The figure below will reveal the situation better:
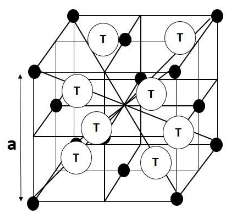
From the figure it is clear that the distance between two tetrahedral voids in a cube is equal to $ \dfrac{\text{a}}{2} $ .
The correct answer here is option C.
Note:
There are two types of voids in the crystal structures: the tetrahedral voids and the octahedral voids. The tetrahedral voids are empty spaces between the particles in a unit cell of a tetrahedral crystal structure, whereas the octahedral voids are the empty spaces between the particles that are present in the unit cell of an octahedral crystal system.
Complete step by step solution:
To answer this question, let us take the example of the Face Centred Cubic Crystal system. There are a total 8 tetrahedral voids in a Face Centred Cubic lattice. Now if we divide the Face Centred Cubic lattice into 8 smaller cubes then each cube will have one tetrahedral void at the centre of the cube at the body centre of the small cubes. Now if we consider the length of the side of the big Face Centred Cube be a, then on breaking into smaller cubes the edge length of each cube will be $ \dfrac{\text{a}}{2} $ .
The figure below will reveal the situation better:

From the figure it is clear that the distance between two tetrahedral voids in a cube is equal to $ \dfrac{\text{a}}{2} $ .
The correct answer here is option C.
Note:
There are two types of voids in the crystal structures: the tetrahedral voids and the octahedral voids. The tetrahedral voids are empty spaces between the particles in a unit cell of a tetrahedral crystal structure, whereas the octahedral voids are the empty spaces between the particles that are present in the unit cell of an octahedral crystal system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




