
The smallest ketone and its net homologue are reacted with \[N{H_2}OH\] to form oxime-
A.Two different oximes are formed
B.Three different oximes are formed
C.Two oximes are optically active
D.All oximes are optically active
Answer
497.1k+ views
Hint: Ketones are the carbonyl compounds that react with hydroxyl amine and undergo a loss of water molecule to form an oxime. The smallest ketone is acetone and the next homologue is butanone. These both ketones reacted to form two oximes and were formed as optically active compounds.
Complete answer:
Organic compounds are of different types. Ketones and aldehydes belong to carbonyl compounds. Ketones consist of a carbonyl group attached to two different carbons or two alkyl groups. The smallest ketone is acetone it has the molecular formula of \[{C_3}{H_6}O\] The next homologue is butanone it has the molecular formula of \[{C_4}{H_8}O\] .
When acetone is treated with hydroxylamine, acetoxime will be formed by the elimination of water molecules.
\[C{H_3}COC{H_3} + N{H_2}OH \to C{H_3}C\left( {NOH} \right)C{H_3}\]
The product is acetoxime. It is an optically active oxime.
When butanone reacts with hydroxylamine, an oxime will be formed by the elimination of water molecules.
\[C{H_3}CO{C_2}{H_5} + N{H_2}OH \to C{H_3}C\left( {NOH} \right){C_2}{H_5}\]
The chemical reaction of the two ketones will be as follows:
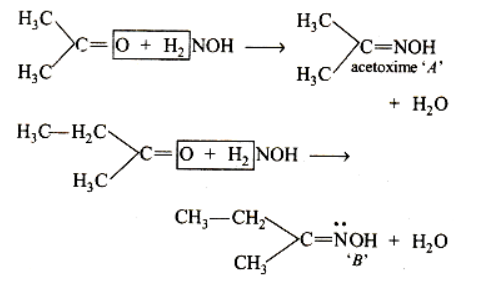
The two oximes contain a different group and do not have a plane of symmetry. Thus, these two oxides are optically active compounds.
Option B is the correct one.
Note:
The above oxime B has lone pair of electrons on nitrogen atom. This lone pair of electrons leads to the formation of two geometrical isomers. Geometrical isomers have a different stereo arrangement around the carbon atom, oxime B has two different stereo arrangements.
Complete answer:
Organic compounds are of different types. Ketones and aldehydes belong to carbonyl compounds. Ketones consist of a carbonyl group attached to two different carbons or two alkyl groups. The smallest ketone is acetone it has the molecular formula of \[{C_3}{H_6}O\] The next homologue is butanone it has the molecular formula of \[{C_4}{H_8}O\] .
When acetone is treated with hydroxylamine, acetoxime will be formed by the elimination of water molecules.
\[C{H_3}COC{H_3} + N{H_2}OH \to C{H_3}C\left( {NOH} \right)C{H_3}\]
The product is acetoxime. It is an optically active oxime.
When butanone reacts with hydroxylamine, an oxime will be formed by the elimination of water molecules.
\[C{H_3}CO{C_2}{H_5} + N{H_2}OH \to C{H_3}C\left( {NOH} \right){C_2}{H_5}\]
The chemical reaction of the two ketones will be as follows:

The two oximes contain a different group and do not have a plane of symmetry. Thus, these two oxides are optically active compounds.
Option B is the correct one.
Note:
The above oxime B has lone pair of electrons on nitrogen atom. This lone pair of electrons leads to the formation of two geometrical isomers. Geometrical isomers have a different stereo arrangement around the carbon atom, oxime B has two different stereo arrangements.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




