
The spectrum from black body radiation is
A) a Line spectrum
B) a band spectrum
C) a continuous spectrum
D) Line and Band spectrum
Answer
586.2k+ views
Hint: Use the theory of continuous spectrum and Stefan Boltzmann Law. The spectrum has a continuous wavelength. We know that a spectrum that has many wavelengths without gaps over a wide range of wavelengths thus appearing to be continuous is known as a continuous spectrum. Thus, the spectrum emitted by a black body is a continuous spectrum because a black body can emit a variety of wavelengths depending upon the black body's temperature.
Complete step by step answer:
Stefan Boltzmann law:
According to the Boltzmann law, the amount of radiation emitted per unit area of the black body at absolute temperature is directly proportional to the fourth power of the temperature.
$E = \sigma {T^4}$
Where $T$ is the temperature
$E$ is the energy
$\sigma $ is the Stefan Boltzmann constant
$\sigma $ has the value of $5.6704 \times {10^5} J c{m^{-2}}{s^{-1}}{K^{-4}}$
The graph given here is plotted between temperature and energy. It is clear that from the graph if the temperature increases the energy also increases.
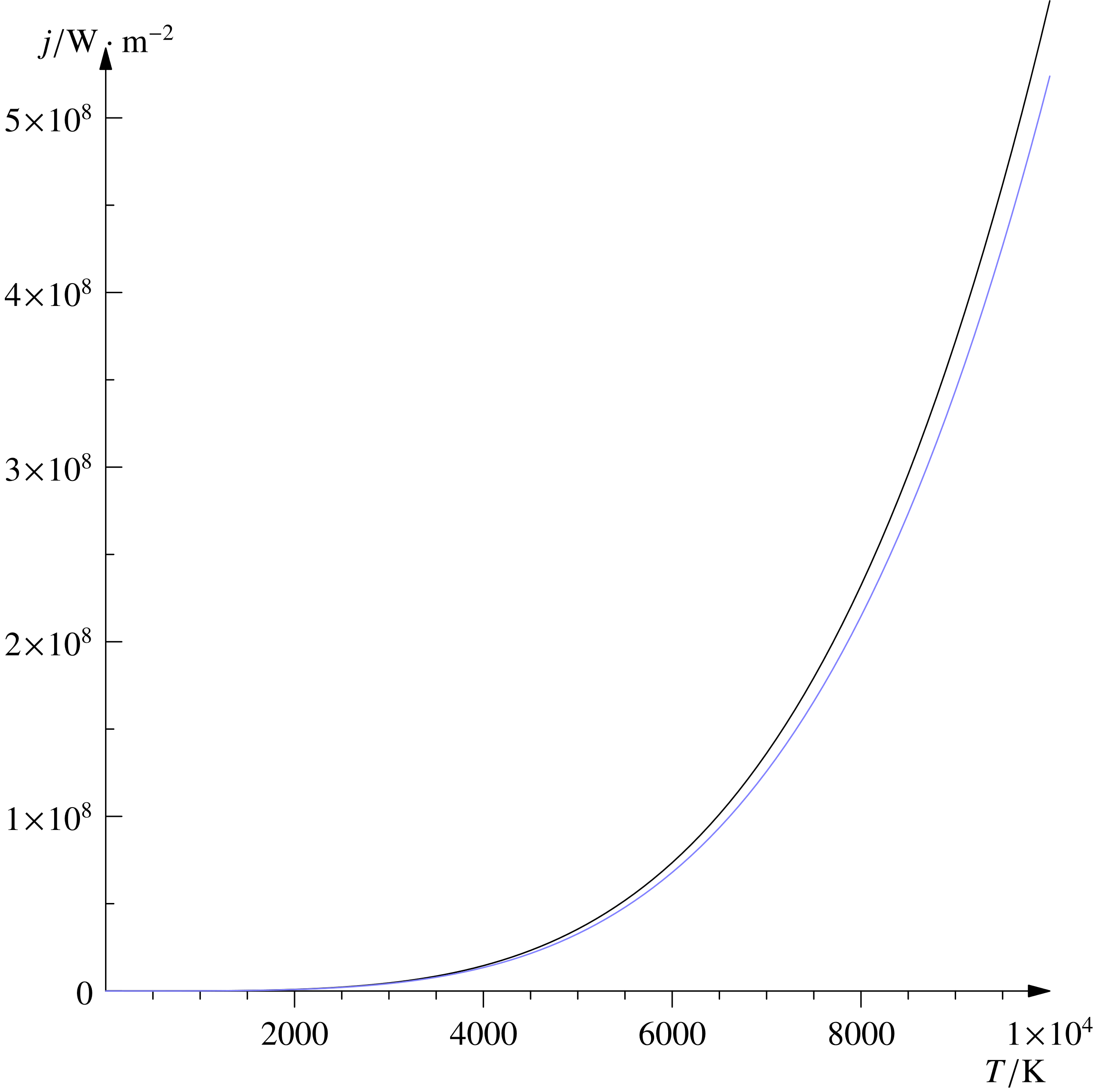
Therefore, the correct answer is an option (C).
Note:
Wien's displacement law states that the wavelength that carries the maximum energy will be inversely proportional to the absolute temperature of a black body. Also, all bodies above $0K$, the absolute temperature, will emit thermal radiation. Anybody at a particular temperature will radiate energy waves. These energy waves may be of different wavelengths, but the waves that are emitted will conjoin to form a spectrum of wavelengths. The wavelength that emits the maximum energy at a specific temperature is known as the maximum wavelength. Therefore, Wien's displacement law can also be interpreted as a product between a particular temperature and the maximum wavelength emitted at that temperature.
Hence, we can conclude that as the temperature rises, the maximum wavelength emitted becomes shorter.
Line Spectra, also known as the atomic Spectra since the lines look like the wavelengths that are radiated from the atoms when the electrons change their levels of energy.
Band Spectra or molecular Spectra is the name that is given to groups of lines that are so close to each other that each group looks like a band. They are formed when molecules emit either their rotational or vibrational energies or emit both energies simultaneously.
Complete step by step answer:
Stefan Boltzmann law:
According to the Boltzmann law, the amount of radiation emitted per unit area of the black body at absolute temperature is directly proportional to the fourth power of the temperature.
$E = \sigma {T^4}$
Where $T$ is the temperature
$E$ is the energy
$\sigma $ is the Stefan Boltzmann constant
$\sigma $ has the value of $5.6704 \times {10^5} J c{m^{-2}}{s^{-1}}{K^{-4}}$
The graph given here is plotted between temperature and energy. It is clear that from the graph if the temperature increases the energy also increases.

Therefore, the correct answer is an option (C).
Note:
Wien's displacement law states that the wavelength that carries the maximum energy will be inversely proportional to the absolute temperature of a black body. Also, all bodies above $0K$, the absolute temperature, will emit thermal radiation. Anybody at a particular temperature will radiate energy waves. These energy waves may be of different wavelengths, but the waves that are emitted will conjoin to form a spectrum of wavelengths. The wavelength that emits the maximum energy at a specific temperature is known as the maximum wavelength. Therefore, Wien's displacement law can also be interpreted as a product between a particular temperature and the maximum wavelength emitted at that temperature.
Hence, we can conclude that as the temperature rises, the maximum wavelength emitted becomes shorter.
Line Spectra, also known as the atomic Spectra since the lines look like the wavelengths that are radiated from the atoms when the electrons change their levels of energy.
Band Spectra or molecular Spectra is the name that is given to groups of lines that are so close to each other that each group looks like a band. They are formed when molecules emit either their rotational or vibrational energies or emit both energies simultaneously.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




