
The stability of the ferric ion is due to:
A) half-filled d-orbitals
B) half-filled f-orbitals
C) completely filled d-orbitals
D) completely filled f-orbitals
Answer
535.5k+ views
Hint: Polyacrylonitrile is a polymer. A molecule that can react with other molecules to form very large molecules having high molecular weights is known as a monomer. The high molecular weight molecules formed are known as polymers. The monomer is a repeating unit of polymer.
Complete step-by-step answer:
Iron is the 3d series element having an atomic number is 26, which indicates there are 26 electrons are there in the iron. There are two main ions formed by the iron: ferric and ferrous ions. The ferric ion is \[{\text{F}}{{\text{e}}^{3 + }}\] and ferrous ion is \[{\text{F}}{{\text{e}}^{2 + }}\]. These ions are formed by the loss of the valence electrons from the neutral iron atom.
As we know the electronic configuration of the iron is represented as follows:
\[{\text{Fe}}\,\,\,\,\,\left[ {{\text{Ar}}} \right]{\text{3}}{{\text{d}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{2}}}\]
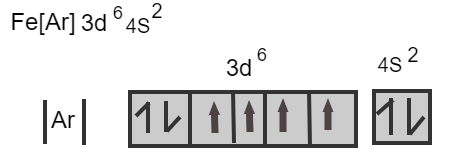
Iron losses three electrons from its neutral atom leads to the form \[{\text{F}}{{\text{e}}^{3 + }}\] ion known as a ferric ion.
\[{\text{Fe}}{\,^{ + 3}}\,\,\,\,\left[ {{\text{Ar}}} \right]{\text{3}}{{\text{d}}^5}{\text{4}}{{\text{s}}^0}\]
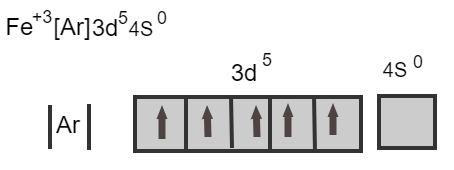
Here, two electrons from the 4s orbital and one electron from the 3d orbital is removed and there are five electrons in the d orbital. The maximum capacity of the d orbital is ten electrons, as there are five electrons it is a half-filled orbital.
If the valence orbital of an atom or ion is either completely filled or half-filled such an ion or atom is stable. Here, in the case of the ferric ion d orbital is half-filled which gives stability to the ferric ion.
Therefore, option (A) is the correct answer to the question. Now, in option (B) it is given that half-filled f orbitals is an incorrect option because there are no f orbitals in the ferric ion.
Here, option (C) is also incorrect as in ferric ion there are no completely filled d orbitals.Now, option (D) is also incorrect because of the absence of the f orbitals in the ferric ion.
Note: In most of the ions or atoms stable electronic configuration is the noble gas electronic configuration in which all the valence orbitals are either completely filled. In some ions, the extra stability of the ion is attained because of the half-filled valence orbitals. In this way ions or elements with either half-filled or completely filled orbitals are stable.
Complete step-by-step answer:
Iron is the 3d series element having an atomic number is 26, which indicates there are 26 electrons are there in the iron. There are two main ions formed by the iron: ferric and ferrous ions. The ferric ion is \[{\text{F}}{{\text{e}}^{3 + }}\] and ferrous ion is \[{\text{F}}{{\text{e}}^{2 + }}\]. These ions are formed by the loss of the valence electrons from the neutral iron atom.
As we know the electronic configuration of the iron is represented as follows:
\[{\text{Fe}}\,\,\,\,\,\left[ {{\text{Ar}}} \right]{\text{3}}{{\text{d}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{2}}}\]

Iron losses three electrons from its neutral atom leads to the form \[{\text{F}}{{\text{e}}^{3 + }}\] ion known as a ferric ion.
\[{\text{Fe}}{\,^{ + 3}}\,\,\,\,\left[ {{\text{Ar}}} \right]{\text{3}}{{\text{d}}^5}{\text{4}}{{\text{s}}^0}\]

Here, two electrons from the 4s orbital and one electron from the 3d orbital is removed and there are five electrons in the d orbital. The maximum capacity of the d orbital is ten electrons, as there are five electrons it is a half-filled orbital.
If the valence orbital of an atom or ion is either completely filled or half-filled such an ion or atom is stable. Here, in the case of the ferric ion d orbital is half-filled which gives stability to the ferric ion.
Therefore, option (A) is the correct answer to the question. Now, in option (B) it is given that half-filled f orbitals is an incorrect option because there are no f orbitals in the ferric ion.
Here, option (C) is also incorrect as in ferric ion there are no completely filled d orbitals.Now, option (D) is also incorrect because of the absence of the f orbitals in the ferric ion.
Note: In most of the ions or atoms stable electronic configuration is the noble gas electronic configuration in which all the valence orbitals are either completely filled. In some ions, the extra stability of the ion is attained because of the half-filled valence orbitals. In this way ions or elements with either half-filled or completely filled orbitals are stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




