
What will be the structural formula of But-2-ene?
Answer
501.3k+ views
Hint: But-2-ene is also called 1,2-dimethylethylene. Structural formula shows how the various atoms are bonded to each other. They are all connected by individual lines. Structural formulas can be easily drawn if we know the chemical formula.
Complete answer:
First we will look at the chemical formula of But-2-ene.
‘But’ is mentioned hence it is a four carbon compound and also ‘ene’ is mentioned it means that it is an alkene. Hence there will be a presence of a double bond. Also , but-2-ene shows isomerism(geometrical isomerism).
There are 2 types of geometrical isomers:
Cis-isomer: These molecules have side groups attached on the same side of the double bond.
Trans-isomer: In these molecules, side groups are attached on opposite sides of the double bond.
Let’s look at its structure :
Normally we would represent its structure as:
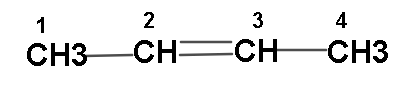
(In this structure we have attached a double bond at the second carbon as 2-ene has been mentioned.)
Now we will give the structures of its 2 isomers.
First we will look at cis-but-2-ene: Clearly in this image side groups are on the same side, hence it is cis-but-2-ene.
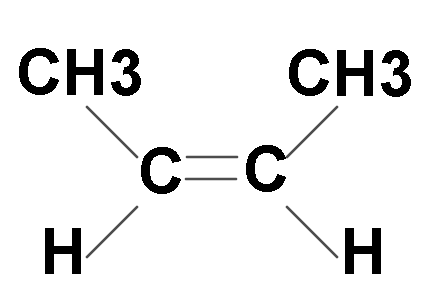
Now let us look at its trans isomer: In this structure side groups are on opposite sides.
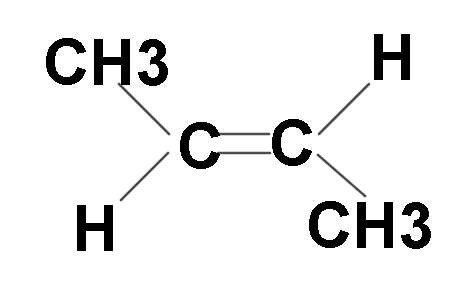
Hence these are the 2 possible structures of But-2-ene.
Note:
Do not just write structure without showing its isomers, it would not n=be correct, show all the possible isomers of a compound. Remember that cis isomers and trans isomers are only true for double bonded carbon compounds(alkenes).
Complete answer:
First we will look at the chemical formula of But-2-ene.
‘But’ is mentioned hence it is a four carbon compound and also ‘ene’ is mentioned it means that it is an alkene. Hence there will be a presence of a double bond. Also , but-2-ene shows isomerism(geometrical isomerism).
There are 2 types of geometrical isomers:
Cis-isomer: These molecules have side groups attached on the same side of the double bond.
Trans-isomer: In these molecules, side groups are attached on opposite sides of the double bond.
Let’s look at its structure :
Normally we would represent its structure as:

(In this structure we have attached a double bond at the second carbon as 2-ene has been mentioned.)
Now we will give the structures of its 2 isomers.
First we will look at cis-but-2-ene: Clearly in this image side groups are on the same side, hence it is cis-but-2-ene.

Now let us look at its trans isomer: In this structure side groups are on opposite sides.

Hence these are the 2 possible structures of But-2-ene.
Note:
Do not just write structure without showing its isomers, it would not n=be correct, show all the possible isomers of a compound. Remember that cis isomers and trans isomers are only true for double bonded carbon compounds(alkenes).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




