
The structure A represents Wedge-Dash Notation of a compound. The structures $I$ to $IV$ represents Fischer Projections for the same compound. Which Fischer Projection is the correct representation of structure A?
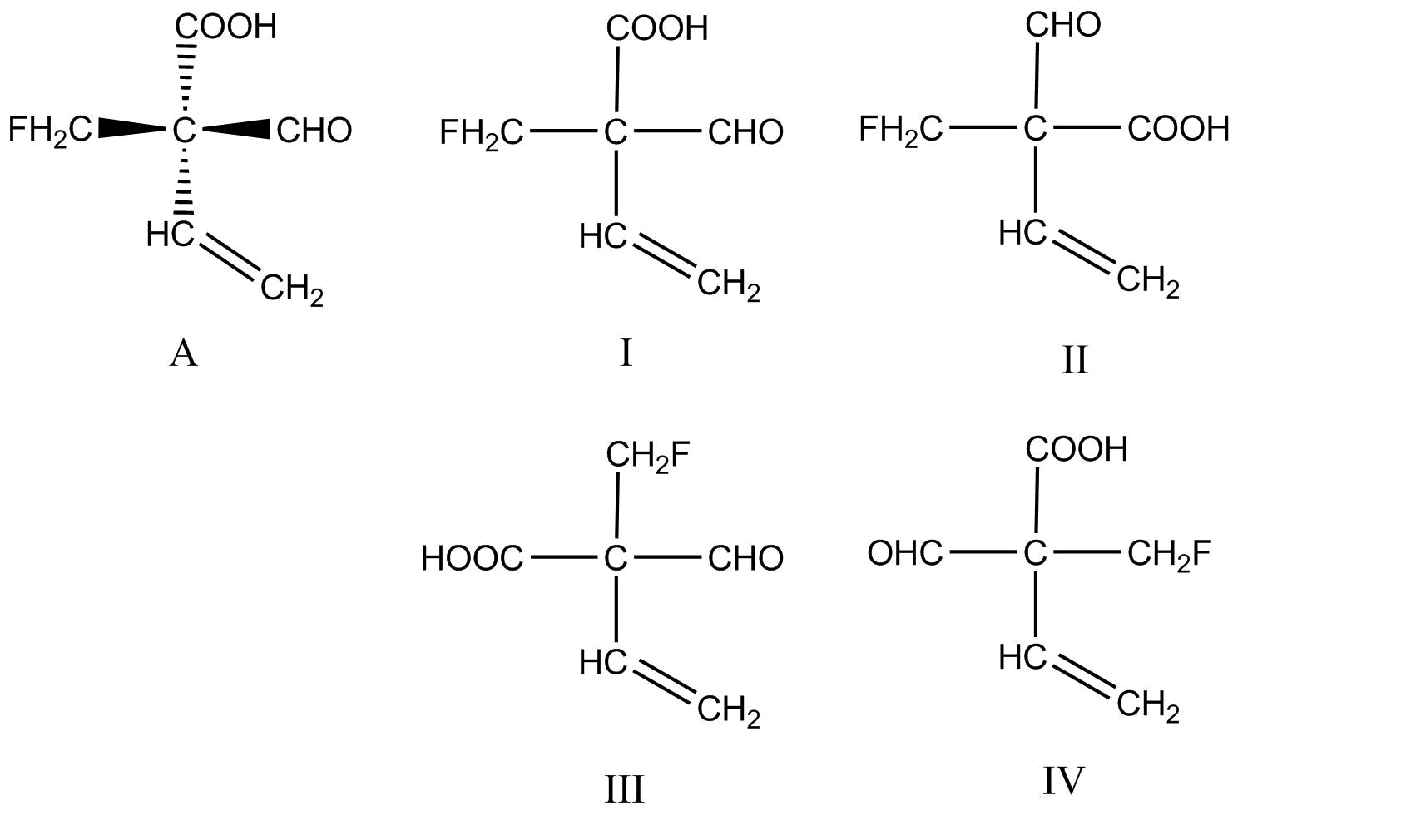
A.$I$
B.$II$
C.$III$
D.$IV$

Answer
563.4k+ views
Hint:The only difference between fischer notation and dash and wedge notation is that, in dash and wedge notation, the bonds which are in the plane are represented with a line, and the bonds which are out of the plane towards us are represented by wedges, and the bonds which are inside the plane away from us are drawn in dashes.
Whereas in case of fischer projection, all the bonds are shown by using lines or sticks.
Complete step by step answer:
The most generally used notation for the representation of straight-chain molecules in the field of organic chemistry is the Wedge-Dash Notation. In this type of notation, two bonds are drawn in the plane of the page in which we are writing (sticks), one bond is drawn coming toward us, that is out of the plane of page (wedged), and one bond is shown going away from us, which is behind the plane of the page (dashed). All the three types of bond represented in this notation differ from each other in terms of spatial arrangement, by considering the page as the plane of the molecule.
On the other hand, the fischer projection can be defined as a two-dimensional representation of an organic molecule which is present in three-dimensional projection. Fischer projections were originally proposed for the representation of carbohydrates and originally used by chemists, specifically in biochemistry and organic chemistry.
Now, we know that if we compare both of these notations, only the type of representation will change and not the structure of the compound. In other words, the functional groups will remain in their original positions as it is, only the dashes and wedges wouldn’t be shown, instead they will be represented by lines only.
If you consider the structure $I$, we can see clearly that it resembles the exact structure of compound A, where all the groups are in the same position.
In the rest of the structures, the position of functional groups are changed, so the correct structure would be the first one.
Hence the correct option is A.
Note:
In a Fischer Projection, the vertical lines which are oriented away from us and the horizontal lines are oriented toward us. The group which is most oxidized is placed at the top.
In case of Wedge-Dash Notation for three-Dimensional Representation of an organic molecule, the dashes signifies a bond which is below the plane of the paper. The wedges signifies a bond which is present above the plane of the paper. The remaining two bonds which are represented by solid lines are in the plane of the paper.
Whereas in case of fischer projection, all the bonds are shown by using lines or sticks.
Complete step by step answer:
The most generally used notation for the representation of straight-chain molecules in the field of organic chemistry is the Wedge-Dash Notation. In this type of notation, two bonds are drawn in the plane of the page in which we are writing (sticks), one bond is drawn coming toward us, that is out of the plane of page (wedged), and one bond is shown going away from us, which is behind the plane of the page (dashed). All the three types of bond represented in this notation differ from each other in terms of spatial arrangement, by considering the page as the plane of the molecule.
On the other hand, the fischer projection can be defined as a two-dimensional representation of an organic molecule which is present in three-dimensional projection. Fischer projections were originally proposed for the representation of carbohydrates and originally used by chemists, specifically in biochemistry and organic chemistry.
Now, we know that if we compare both of these notations, only the type of representation will change and not the structure of the compound. In other words, the functional groups will remain in their original positions as it is, only the dashes and wedges wouldn’t be shown, instead they will be represented by lines only.
If you consider the structure $I$, we can see clearly that it resembles the exact structure of compound A, where all the groups are in the same position.
In the rest of the structures, the position of functional groups are changed, so the correct structure would be the first one.
Hence the correct option is A.
Note:
In a Fischer Projection, the vertical lines which are oriented away from us and the horizontal lines are oriented toward us. The group which is most oxidized is placed at the top.
In case of Wedge-Dash Notation for three-Dimensional Representation of an organic molecule, the dashes signifies a bond which is below the plane of the paper. The wedges signifies a bond which is present above the plane of the paper. The remaining two bonds which are represented by solid lines are in the plane of the paper.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




