
The structure of ${H_2}{O_2}$ is:
A. Planar
B. Non-Planar
C. Spherical
D. Linear
Answer
543.9k+ views
Hint: Hydrogen peroxide is known for an open book structure with O – O spins. 2 planes are there in this structure and each plane contains 1 O-H bond pair, and the angle b/w both the planes is 90.2°.
Complete step-by-step answer: Hydrogen peroxide has a structure which is non-planar. ${H_2}{O_2}$ is known as an open book structure with O – O spins. The dihedral angle of this structure is 111°. The O-O bond length equal to 145.8 pm and the O-H bond length equal to 98.8 pm (which is actually 9.88 × 10-13 m).
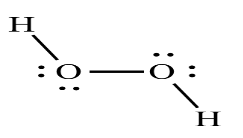
So it is visible that the structure of ${H_2}{O_2}$ will surely cause the net dipole moment to move near the O atom, however since the molecule has a bent structure, the dipole moments will not cancel out, which means ${H_2}{O_2}$ is a polar molecule.
Additional information:
Hydrogen Peroxide is an oxidizing agent along with disinfectant, antiviral and anti-bacterial functionalities. On rinsing and gargling or topical application, hydrogen peroxide exerts its oxidizing activity and forms free radicals that results in oxidative damage to proteins and membrane lipids.
Even swallowing very small amounts of household around 3% hydrogen peroxide usually is not harmful. However it can create a lot of foam. Household hydrogen peroxide can create irritation to the eyes and skin. And the higher concentrations can cause burns.
As it is not advisable to be used for large open wounds or deep cuts, or for a long time. Hydrogen peroxide functions by killing bacteria, whether it is “good” healing bacteria or “bad” infection-causing bacteria.
Hence, Option B is correct.
Note: As Hydrogen peroxide is not linear in shape and has an open book structure with O-O spins, so no confusion should be left for choosing the correct option which is non-linear as mentioned above.
Complete step-by-step answer: Hydrogen peroxide has a structure which is non-planar. ${H_2}{O_2}$ is known as an open book structure with O – O spins. The dihedral angle of this structure is 111°. The O-O bond length equal to 145.8 pm and the O-H bond length equal to 98.8 pm (which is actually 9.88 × 10-13 m).
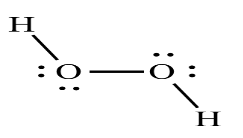
So it is visible that the structure of ${H_2}{O_2}$ will surely cause the net dipole moment to move near the O atom, however since the molecule has a bent structure, the dipole moments will not cancel out, which means ${H_2}{O_2}$ is a polar molecule.
Additional information:
Hydrogen Peroxide is an oxidizing agent along with disinfectant, antiviral and anti-bacterial functionalities. On rinsing and gargling or topical application, hydrogen peroxide exerts its oxidizing activity and forms free radicals that results in oxidative damage to proteins and membrane lipids.
Even swallowing very small amounts of household around 3% hydrogen peroxide usually is not harmful. However it can create a lot of foam. Household hydrogen peroxide can create irritation to the eyes and skin. And the higher concentrations can cause burns.
As it is not advisable to be used for large open wounds or deep cuts, or for a long time. Hydrogen peroxide functions by killing bacteria, whether it is “good” healing bacteria or “bad” infection-causing bacteria.
Hence, Option B is correct.
Note: As Hydrogen peroxide is not linear in shape and has an open book structure with O-O spins, so no confusion should be left for choosing the correct option which is non-linear as mentioned above.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




