
The structure of $IBr_{2}^{-}$ involves hybridization of the type:
(A) $s{{p}^{3}}$
(B) $s{{p}^{3}}d$
(C) $s{{p}^{3}}{{d}^{2}}$
(D) None of these
Answer
585.3k+ views
Hint: The structure of the $IBr_{2}^{-}$ is linear and contains three lone pair of electrons in equatorial position to minimize the lone pair repulsions and the two bromine atoms are located in an axial position. So, the structure of $IBr_{2}^{-}$ is linear.
Complete step by step solution:
-The electronic configuration of iodine is as follows.
\[[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}\]
-Iodine contains 5 valence electrons in a 5p orbital.
-The structure of the $IBr_{2}^{-}$ is as follows.
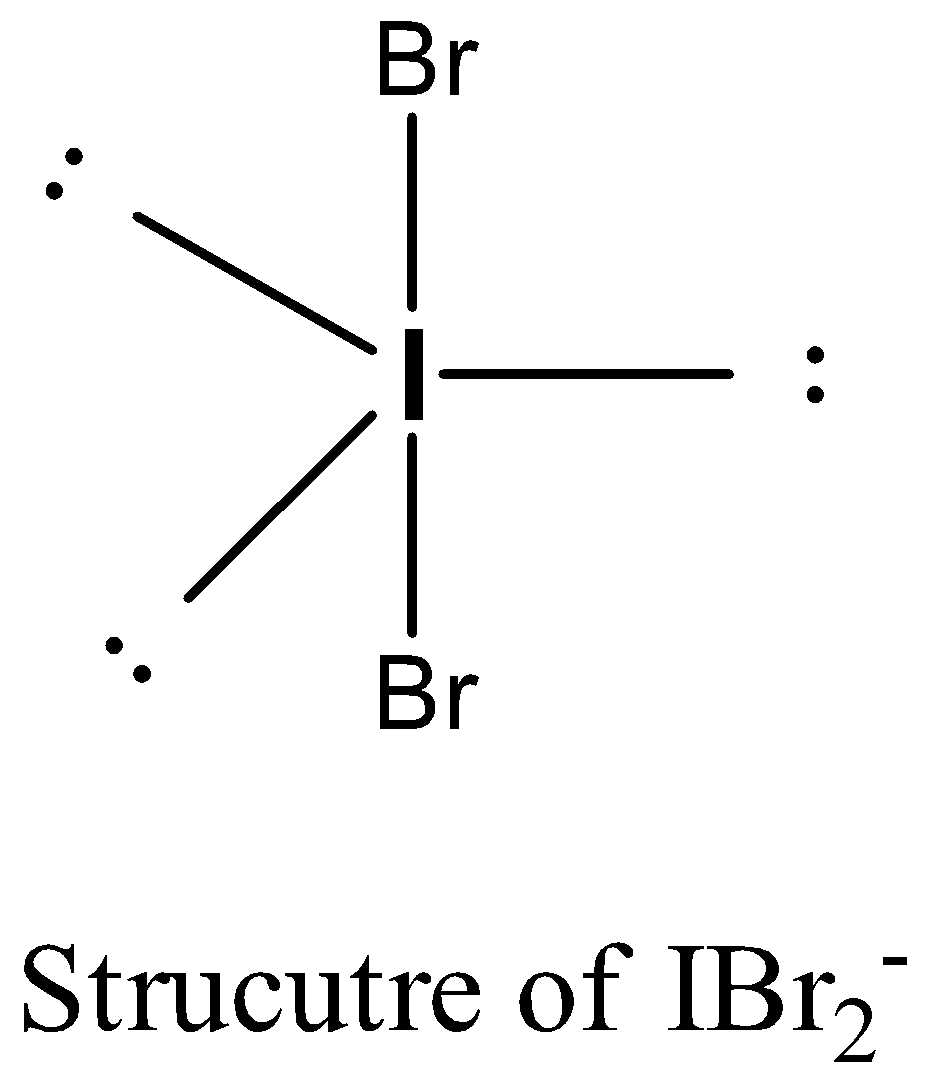
-The hybridization of $IBr_{2}^{-}$ is as follows.
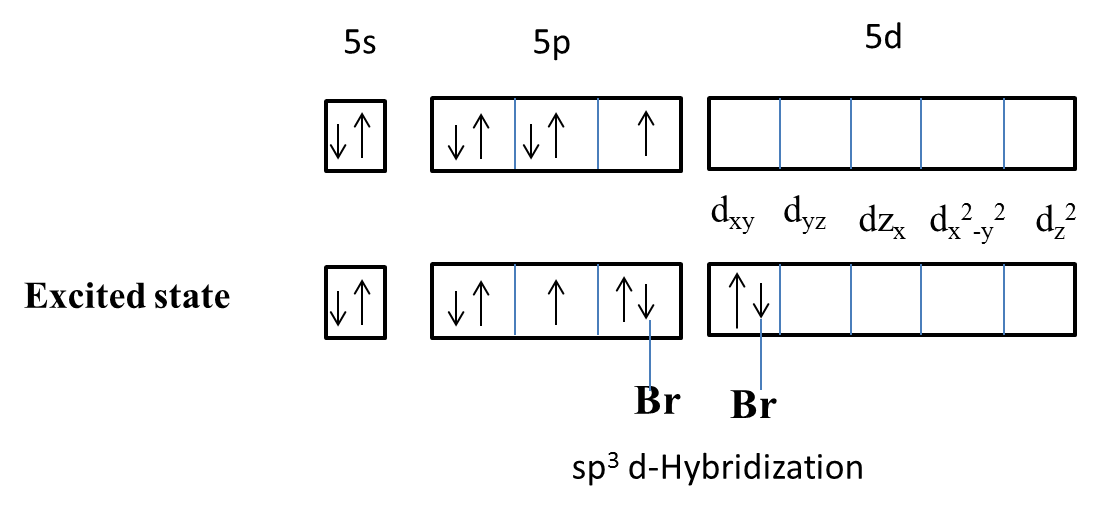
-Iodine undergoes the above type of hybridization of orbitals with bromine atoms.
-Therefore the structure of $IBr_{2}^{-}$ involves hybridization of the type is $s{{p}^{3}}d$ .
So, the correct option is (B).
Additional information:
-The iodine in $IBr_{2}^{-}$ has 2 bond pairs and three lone pair of electrons.
-The two bond pairs are with two bromine atoms.
-According to the bent rule for $s{{p}^{3}}d$ hybridization all lone pair of electrons should be in an equatorial position to minimize the repulsions between lone pair and lone pair.
-Lone pair – lone pair repulsions are very high when compared to lone pair-bond pair and bond pair-bond pair repulsions.
-Therefore the structure of $IBr_{2}^{-}$ is linear.
Note: In the hybridization of $s{{p}^{3}}d$ there is an involvement of one s- orbital, three p- orbitals and one d- orbital. The percentage of s character is 20%, p character is 60% and d character is 20% in $s{{p}^{3}}d$ hybridization.
Complete step by step solution:
-The electronic configuration of iodine is as follows.
\[[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}\]
-Iodine contains 5 valence electrons in a 5p orbital.
-The structure of the $IBr_{2}^{-}$ is as follows.

-The hybridization of $IBr_{2}^{-}$ is as follows.

-Iodine undergoes the above type of hybridization of orbitals with bromine atoms.
-Therefore the structure of $IBr_{2}^{-}$ involves hybridization of the type is $s{{p}^{3}}d$ .
So, the correct option is (B).
Additional information:
-The iodine in $IBr_{2}^{-}$ has 2 bond pairs and three lone pair of electrons.
-The two bond pairs are with two bromine atoms.
-According to the bent rule for $s{{p}^{3}}d$ hybridization all lone pair of electrons should be in an equatorial position to minimize the repulsions between lone pair and lone pair.
-Lone pair – lone pair repulsions are very high when compared to lone pair-bond pair and bond pair-bond pair repulsions.
-Therefore the structure of $IBr_{2}^{-}$ is linear.
Note: In the hybridization of $s{{p}^{3}}d$ there is an involvement of one s- orbital, three p- orbitals and one d- orbital. The percentage of s character is 20%, p character is 60% and d character is 20% in $s{{p}^{3}}d$ hybridization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




