
The systematic naming of the following cycloalkane is:
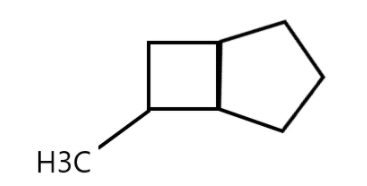
A. 6-Methyl bicyclo [3.2.0] heptane
B. 7-Methyl bicyclo [3.2.0] heptane
C. 2-Methyl bicyclo [3.2.0] heptane
D. 3-Methyl bicyclo [3.2.0] heptane

Answer
582.3k+ views
Hint: In the options you can see that only the first part is different and rest all is the same. You can study the rest part, why any prefix is used and also study the compound. This will help in getting to the answer.
Complete step by step answer:
Starting with revision of cycloalkane, we know that alkanes are compounds having carbon and hydrogen atoms and “cyclo” means having cyclic and closed structure. It means that carbons of the molecule are arranged in the form of rings. Cycloalkanes are saturated meaning that carbon atoms that make up a ring are single bonded to other atoms and there are no double or triple bonds.
Now, observing the compounds we can see that it is an alkane and therefore the suffix –ane will be used. Also, we have two cycloalkanes present in the compound and therefore the prefix bicyclo will be used.
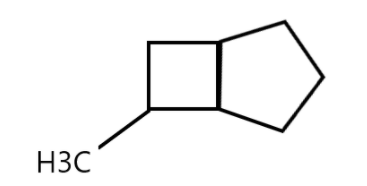
At 6th position we have a methyl group so the keyword methyl is used. Also the reason for using “hept” is because the cyclohexane has 7 vertices. The links contain 3, 2 and 0 carbon atoms and therefore [3.2.0] is used. And finally the methyl group is attached to the cycloalkane in the 6th position and therefore, the compound is called 6-Methyl bicycle [3.2.0] heptane.
So, the correct option is A.
Additional Information:
Cycloalkanes are organic compounds that exist in many products and our body and food and hair products and in every day to day examples. They have a ring like structure formed due to their saturated nature and therefore are more stable in nature. Some uses of cycloalkanes are organic solvent in medical drugs, in the manufacture of hair products, anesthetic agents in the medical field, petroleum industries and many more.
Note:
Sometimes we can make mistakes in numbering the vertices which may lead to wrong IUPAC name. So, we have to be careful in numbering and naming the compound.
Complete step by step answer:
Starting with revision of cycloalkane, we know that alkanes are compounds having carbon and hydrogen atoms and “cyclo” means having cyclic and closed structure. It means that carbons of the molecule are arranged in the form of rings. Cycloalkanes are saturated meaning that carbon atoms that make up a ring are single bonded to other atoms and there are no double or triple bonds.
Now, observing the compounds we can see that it is an alkane and therefore the suffix –ane will be used. Also, we have two cycloalkanes present in the compound and therefore the prefix bicyclo will be used.

At 6th position we have a methyl group so the keyword methyl is used. Also the reason for using “hept” is because the cyclohexane has 7 vertices. The links contain 3, 2 and 0 carbon atoms and therefore [3.2.0] is used. And finally the methyl group is attached to the cycloalkane in the 6th position and therefore, the compound is called 6-Methyl bicycle [3.2.0] heptane.
So, the correct option is A.
Additional Information:
Cycloalkanes are organic compounds that exist in many products and our body and food and hair products and in every day to day examples. They have a ring like structure formed due to their saturated nature and therefore are more stable in nature. Some uses of cycloalkanes are organic solvent in medical drugs, in the manufacture of hair products, anesthetic agents in the medical field, petroleum industries and many more.
Note:
Sometimes we can make mistakes in numbering the vertices which may lead to wrong IUPAC name. So, we have to be careful in numbering and naming the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




