
The third member of the homologous series of aliphatic aldehydes has the structure :
a.) $C{H_3}C{H_2}CHO$
b.) $C{H_3}{(C{H_2})_2}CHO$
c.) $C{H_3}COC{H_2}C{H_3}$
d.) $C{H_3}COC{H_3}$
Answer
589.5k+ views
Hint: The aliphatic compounds are the one that may be linear or cyclic but do not possess the characters of an aromatic compound. In the homologous series, there is an increase in one-carbon unit at each step. The first member is the formaldehyde with one carbon unit. The third member will contain three carbon units.
Complete step by step solution:
First, let us know about the aliphatic compounds.
The hydrocarbons are of two types- the aliphatic and the aromatic.
The aromatic compounds are the one which is cyclic, planar and follow huckel’s rule. The other compounds are the aliphatic one. These may be cyclic or linear chains but do not possess properties of aromatic compounds.
The homologous means that the increasing series.
The first aldehyde is formaldehyde. It has structure-
$HCHO$
In the homologous series, one carbon unit increases at each step. So, the second member of aliphatic aldehyde will be two carbon molecule. The molecule is acetaldehyde. It has structure -
$C{H_3}CHO$
After this is the third member of the homologous series. It will contain three carbon units. The molecule will be propionaldehyde. It will have structure -
$C{H_3}C{H_2}CHO$
It is also called as propanal.
So, the option a.) is the correct answer.
Note: It must be noted that the aldehyde compounds are those that have an aldehydic functional group which is CHO. These compounds are also called carbonyl compounds because of the presence of C=O bond. These groups are terminal in nature. In case, one thinks to put C=O in the centre, then it is called a ketonic group. For example - The three-membered ketone is as-
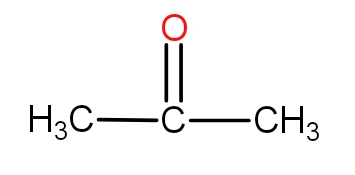
Complete step by step solution:
First, let us know about the aliphatic compounds.
The hydrocarbons are of two types- the aliphatic and the aromatic.
The aromatic compounds are the one which is cyclic, planar and follow huckel’s rule. The other compounds are the aliphatic one. These may be cyclic or linear chains but do not possess properties of aromatic compounds.
The homologous means that the increasing series.
The first aldehyde is formaldehyde. It has structure-
$HCHO$
In the homologous series, one carbon unit increases at each step. So, the second member of aliphatic aldehyde will be two carbon molecule. The molecule is acetaldehyde. It has structure -
$C{H_3}CHO$
After this is the third member of the homologous series. It will contain three carbon units. The molecule will be propionaldehyde. It will have structure -
$C{H_3}C{H_2}CHO$
It is also called as propanal.
So, the option a.) is the correct answer.
Note: It must be noted that the aldehyde compounds are those that have an aldehydic functional group which is CHO. These compounds are also called carbonyl compounds because of the presence of C=O bond. These groups are terminal in nature. In case, one thinks to put C=O in the centre, then it is called a ketonic group. For example - The three-membered ketone is as-

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




