
The total number of compounds having at least one bridging oxo group among the molecules given below is:
${{\text{N}}_2}{{\text{O}}_3},{{\text{N}}_2}{{\text{O}}_5},{{\text{P}}_4}{{\text{O}}_6},{{\text{P}}_4}{{\text{O}}_7},{{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_5},{{\text{H}}_5}{{\text{P}}_3}{{\text{O}}_{10}},{{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_3},{{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_5}$
Answer
586.5k+ views
Hint:Oxo group is the group in which oxygen is attached to another atom with a double bond. The ligands which are bonded to more than one metal atom are called bridging ligands. This results in many poly metallic complexes having new chemical properties.
Complete step by step answer:
When oxygen is connected to another atom with a double bond, then it is known as the oxo group. When the oxo group is attached to more than one metal atom, then it is termed as bridging ligand. Bridging ligands are bonded to each metal atom through one or more donor atoms which form a coordinate covalent bond. Some inorganic bridging ligands are hydroxide ${\text{O}}{{\text{H}}^ - }$, nitride ${{\text{N}}^3}^ - $, carbonyl ${\text{CO}}$, chloride ${\text{C}}{{\text{l}}^ - }$, amido ${\text{N}}{{\text{H}}_2}^ - $, hydride ${{\text{H}}^ - }$, oxide ${{\text{O}}_2}$ etc.
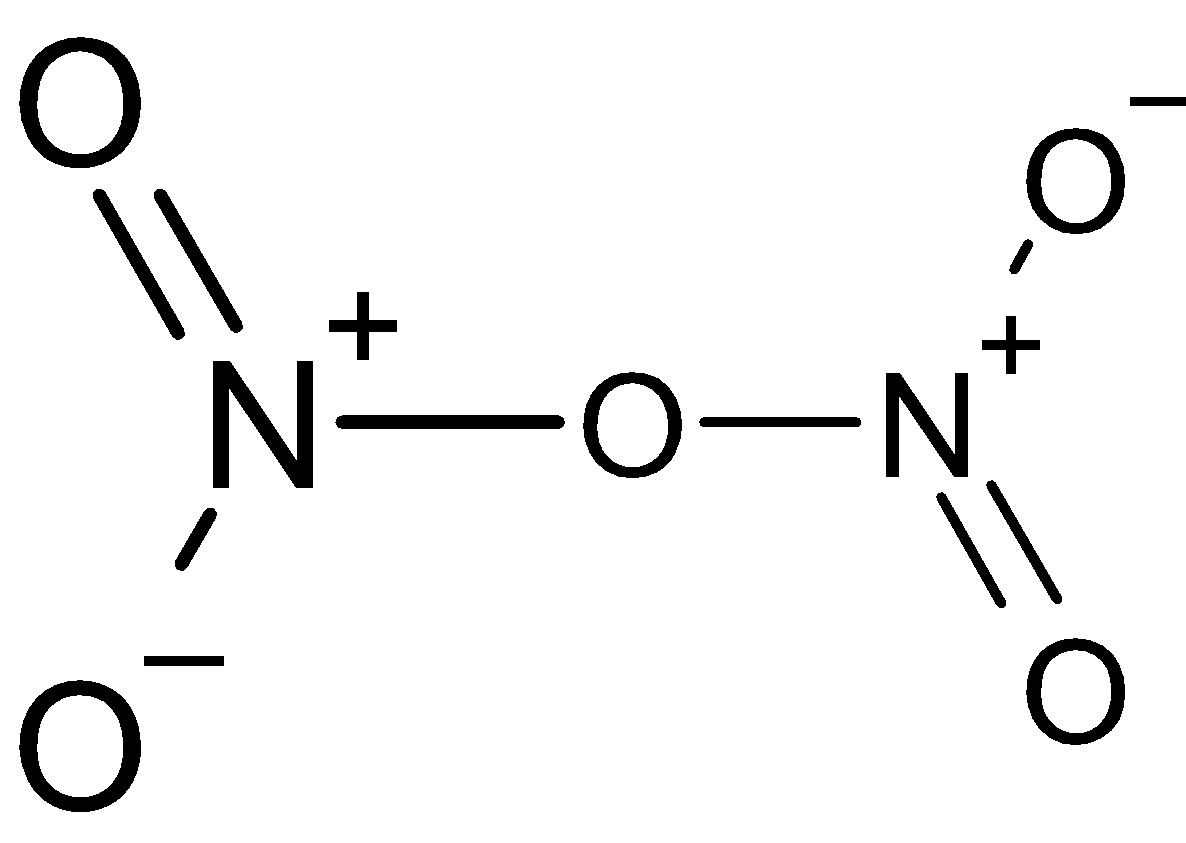
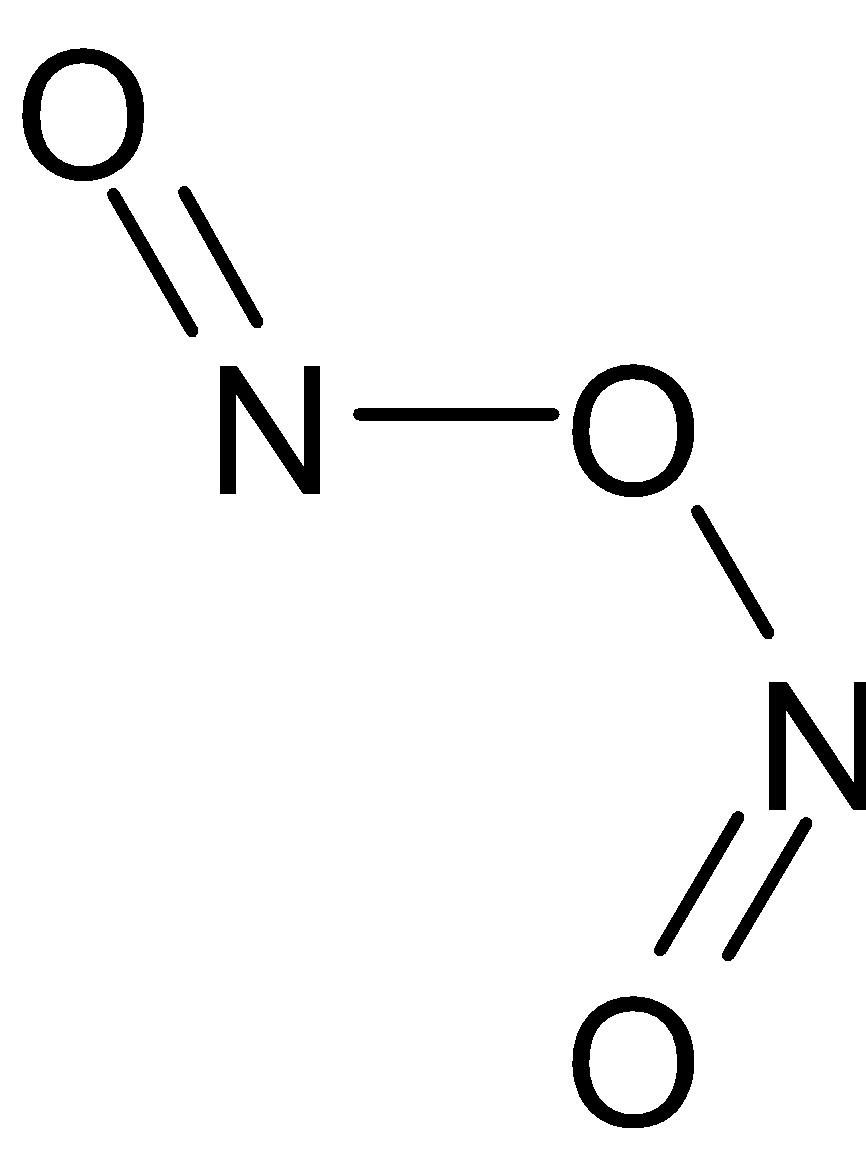
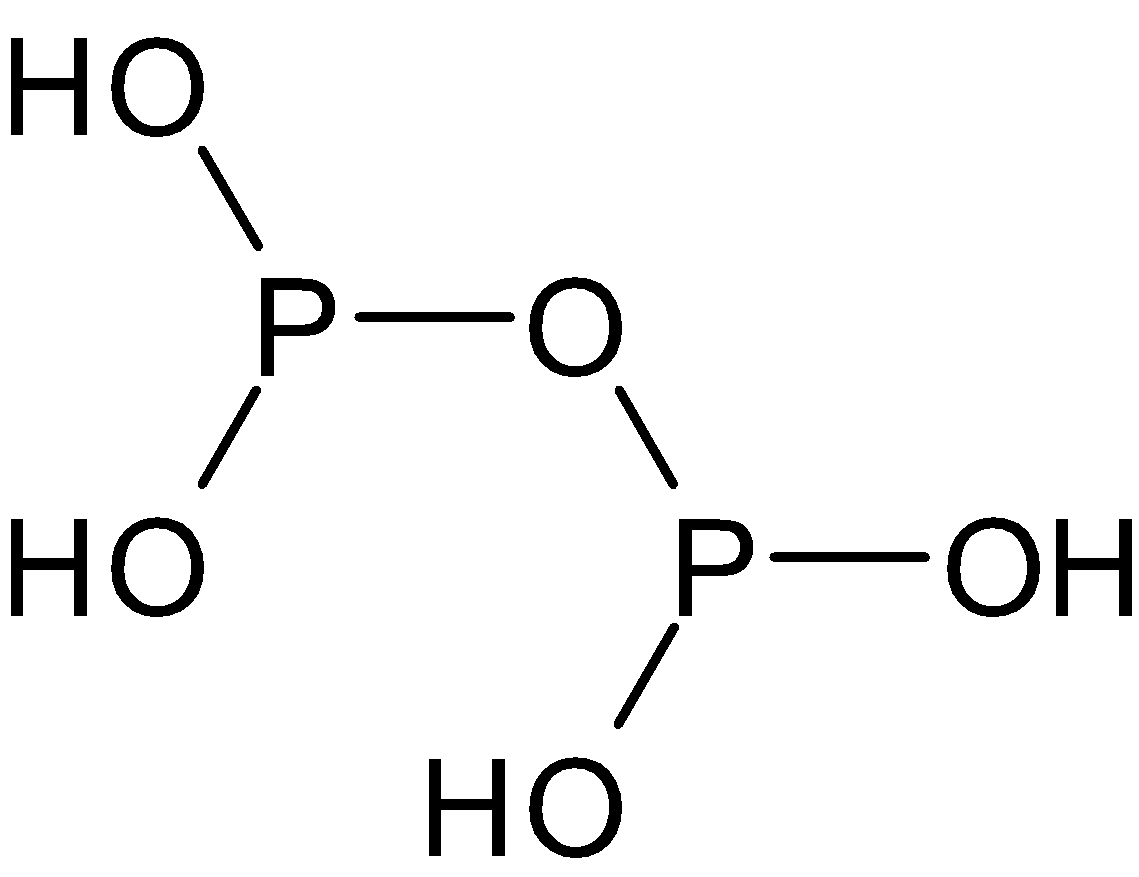
(i) ${{\text{N}}_2}{{\text{O}}_5}$ (ii) ${{\text{N}}_2}{{\text{O}}_3}$ (iii) ${{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_5}$
In (i) the two hydrogen atoms are not there, instead there is only a negative sign for each oxygen atom.
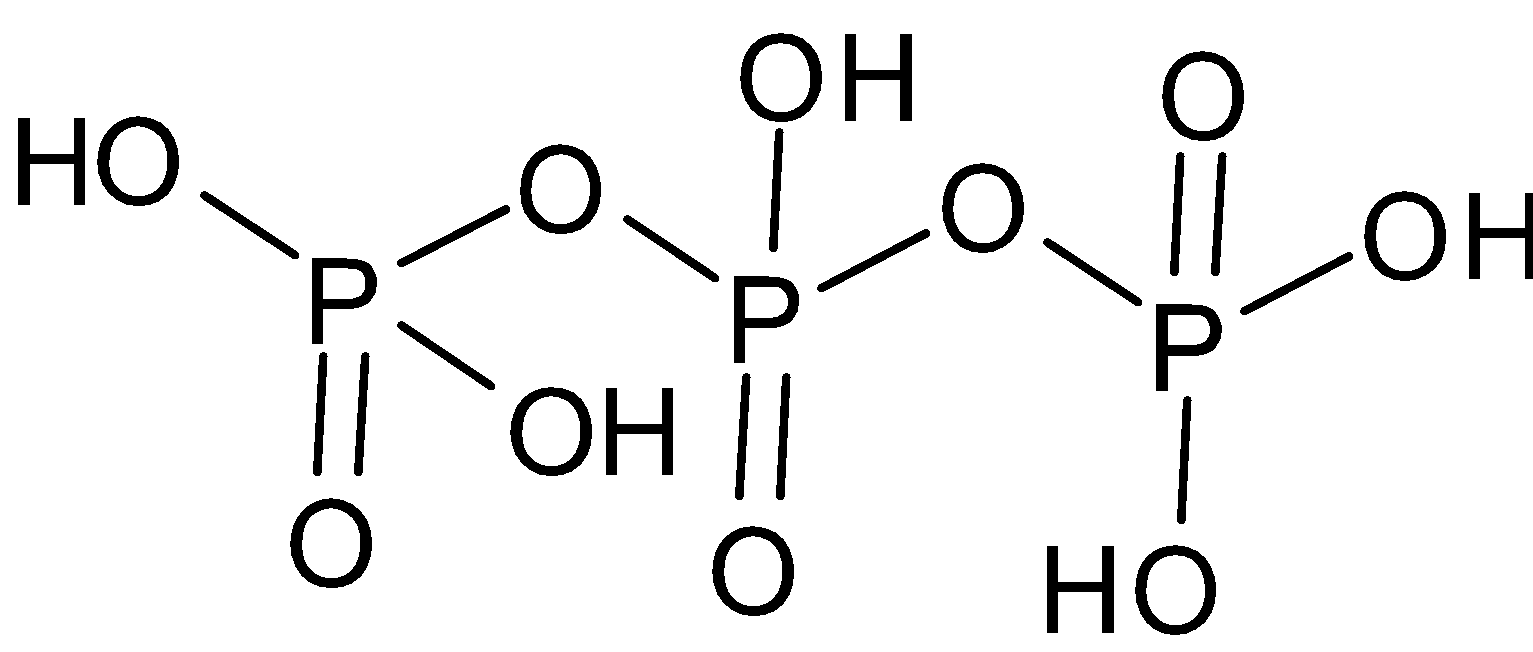
(iv) ${{\text{H}}_5}{{\text{P}}_3}{{\text{O}}_{10}}$
Among the eight compounds, only two of them do not have bridged oxo ligands. They are ${{\text{P}}_4}{{\text{O}}_6}$ and ${{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_5}$. Other six of them have at least one bridged oxo ligand.
Hence the total number of compounds having at least one bridging oxo group is equal to six.
Note:
Bridging ligands are redox and spectroscopically active. Bridged polymetallic complexes show the properties of individual metal complexes from which the complex is formed. Sometimes, the bridging ligands do not lie between the metal atoms.
Complete step by step answer:
When oxygen is connected to another atom with a double bond, then it is known as the oxo group. When the oxo group is attached to more than one metal atom, then it is termed as bridging ligand. Bridging ligands are bonded to each metal atom through one or more donor atoms which form a coordinate covalent bond. Some inorganic bridging ligands are hydroxide ${\text{O}}{{\text{H}}^ - }$, nitride ${{\text{N}}^3}^ - $, carbonyl ${\text{CO}}$, chloride ${\text{C}}{{\text{l}}^ - }$, amido ${\text{N}}{{\text{H}}_2}^ - $, hydride ${{\text{H}}^ - }$, oxide ${{\text{O}}_2}$ etc.
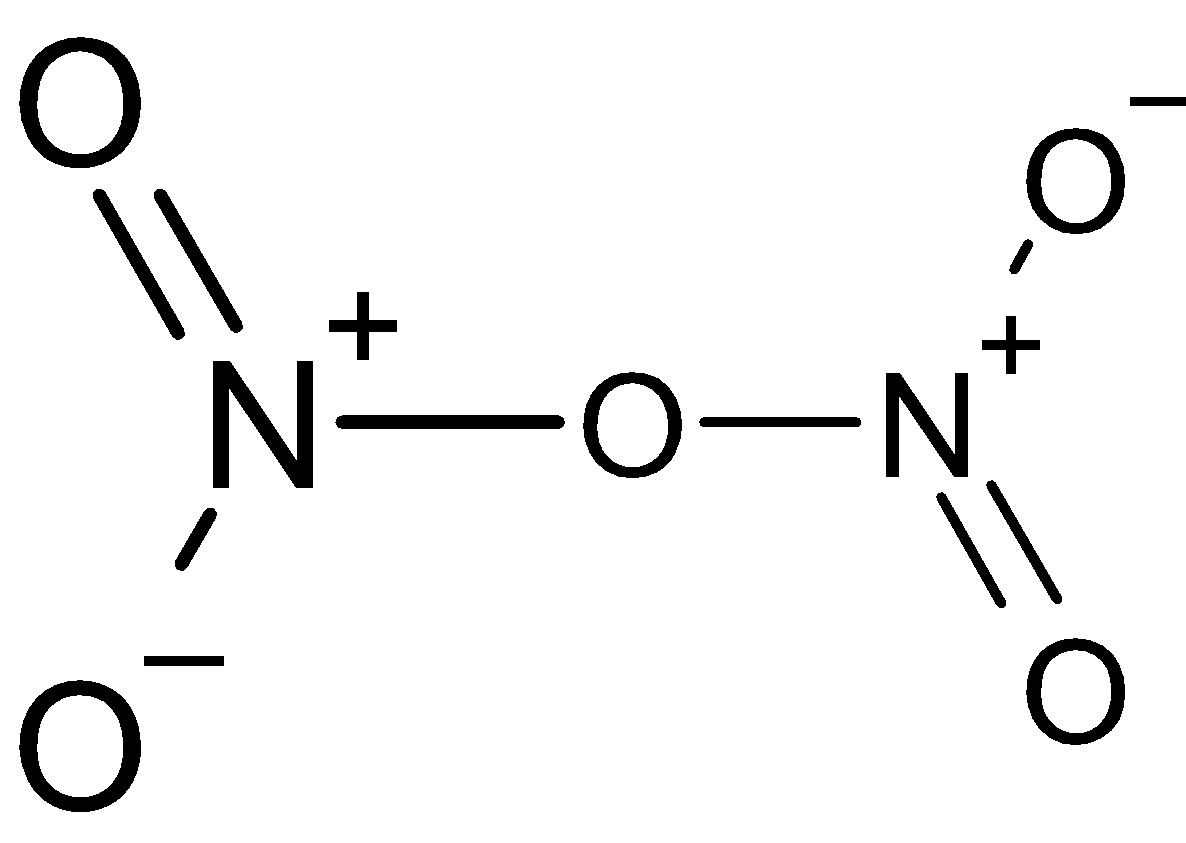


(i) ${{\text{N}}_2}{{\text{O}}_5}$ (ii) ${{\text{N}}_2}{{\text{O}}_3}$ (iii) ${{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_5}$
In (i) the two hydrogen atoms are not there, instead there is only a negative sign for each oxygen atom.

(iv) ${{\text{H}}_5}{{\text{P}}_3}{{\text{O}}_{10}}$
Among the eight compounds, only two of them do not have bridged oxo ligands. They are ${{\text{P}}_4}{{\text{O}}_6}$ and ${{\text{H}}_4}{{\text{P}}_2}{{\text{O}}_5}$. Other six of them have at least one bridged oxo ligand.
Hence the total number of compounds having at least one bridging oxo group is equal to six.
Note:
Bridging ligands are redox and spectroscopically active. Bridged polymetallic complexes show the properties of individual metal complexes from which the complex is formed. Sometimes, the bridging ligands do not lie between the metal atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




