
The total number of lone pairs of electrons in melamine is:
A. Three lone pairs from triazine moiety
B. Three lone pairs from ${{ - N}}{{{H}}_2}$ moiety
C. Six lone pairs from all of the ${{N}}$
D. None of these

Answer
565.8k+ views
Hint: Before calculating the number of lone pairs of electrons in the compound, we have to know about the catenation property of the elements in the given compound. Also, we need to know the valency of each element in the compound so that we can understand the number of electrons gained or lost.
Complete step by step answer:
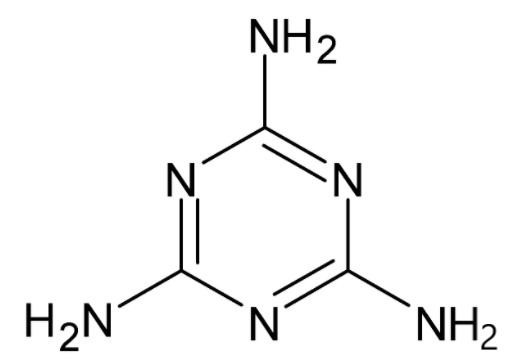
We know that a lone pair of electrons is a pair of electrons which is not bonded with any other atom. Generally, they are present in the valence shell or outermost shell of the atom. They are also termed as non-bonded pairs of electrons. Lewis dot structure is used to find the lone pair of electrons. The structure of melamine is given. Thus the molecular formula of melamine is ${{{C}}_3}{{{H}}_6}{{{N}}_6}$.
Another name for melamine is 1,3,5-triazine. The compound contains carbon, hydrogen and nitrogen atoms. We know that the valencies of carbon, hydrogen and nitrogen are $4,1$ and $5$ respectively, i.e. they have this number of electrons in their valence shell for the bond formation.
In the compound, carbon has satisfied its valency, i.e. it has shared its four valence electrons with hydrogen and nitrogen. Hydrogen also has satisfied its valency. But nitrogen has not satisfied its valency. It has bonded only to three atoms. There is a pair of electrons which is not shared to any other atom. This is known as the lone pair of electrons. All of the nitrogen atoms have a lone pair of electrons. There are a total of six nitrogen atoms. So a total of six lone pairs of electrons are present in the compound.
So the total number of lone pairs of electrons in melamine is six.
Hence, option C is the correct answer.
Note: Melamine can be prepared by reacting urea with cyanuric acid. They have several uses like they have been used as glues, dinnerwares, flame retardants, increase nitrogen content in milk, increase protein content etc.
Complete step by step answer:
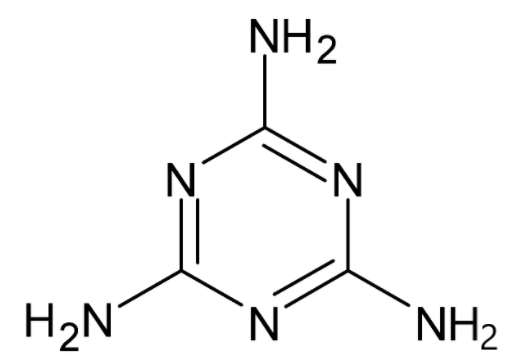
We know that a lone pair of electrons is a pair of electrons which is not bonded with any other atom. Generally, they are present in the valence shell or outermost shell of the atom. They are also termed as non-bonded pairs of electrons. Lewis dot structure is used to find the lone pair of electrons. The structure of melamine is given. Thus the molecular formula of melamine is ${{{C}}_3}{{{H}}_6}{{{N}}_6}$.

Another name for melamine is 1,3,5-triazine. The compound contains carbon, hydrogen and nitrogen atoms. We know that the valencies of carbon, hydrogen and nitrogen are $4,1$ and $5$ respectively, i.e. they have this number of electrons in their valence shell for the bond formation.
In the compound, carbon has satisfied its valency, i.e. it has shared its four valence electrons with hydrogen and nitrogen. Hydrogen also has satisfied its valency. But nitrogen has not satisfied its valency. It has bonded only to three atoms. There is a pair of electrons which is not shared to any other atom. This is known as the lone pair of electrons. All of the nitrogen atoms have a lone pair of electrons. There are a total of six nitrogen atoms. So a total of six lone pairs of electrons are present in the compound.
So the total number of lone pairs of electrons in melamine is six.
Hence, option C is the correct answer.
Note: Melamine can be prepared by reacting urea with cyanuric acid. They have several uses like they have been used as glues, dinnerwares, flame retardants, increase nitrogen content in milk, increase protein content etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




