
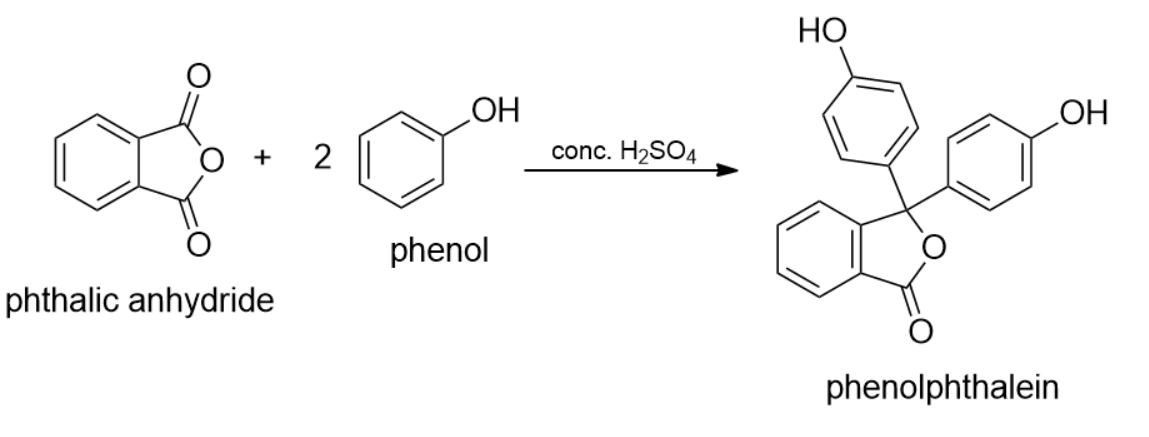
The treatment of phenol with phthalic anhydride in the presence of concentrated sulphuric acid produces:
(A) Methyl orange
(B) Phenolphthalein
(C) Methyl red
(D) Thymol blue
Answer
584.7k+ views
Hint: The reaction between phenol and phthalic anhydride in the presence of concentrated sulphuric acid is a condensation reaction, in which the two reactions combine with the loss of a water molecule.
Complete Step by step answer:
- The reaction between phenol and phthalic anhydride in the presence of sulphuric acid is a well- known reaction for the production of phenolphthalein.
- Sulphuric acid in this reaction acts as a dehydrating catalytic agent. Other agents, which can be used in place of sulfuric acid include anhydrous zinc chloride, anhydrous tin chloride, anhydrous aromatic sulfonic acids.
- Phenolphthalein is renowned as the indicator dye. Phenolphthalein can be synthesized by the condensation of phthalic anhydride with two equivalents of phenol under acidic condition, hence is named as Phenolphthalein by Adolf von Baeyer.
Note: Let us now see the various uses of phenolphthalein indicator:
(i) Use of pH indicator: It is most commonly used as an indicator in acid- base titrations. It also serves as a component or universal indicator when combined with methyl red, bromothymol blue, and thymol blue. Phenolphthalein acquires four different colors in different states of an aqueous solution. It is naturally colorless and remains colorless in acidic pH but turns pink in alkaline aqueous solutions.
(ii) Concrete carbonation: Concrete has naturally high pH due to the calcium hydroxide forms when Portland cement reacts with water. When a 1% solution of phenolphthalein is added to normal concrete, it turns pink, however, it remains colorless if the concrete has undergone carbonation.
(iii) Education: It shows a change of color from colorless to pink and is used in chemistry classes for the study of reaction kinetics.
(iv) Entertainment: It is also used as a component of disappearing inks or disappearing dyes on the Hollywood Barbie hair.
(v) Medical uses: It has been used as a laxative for a century. A laxative is the substance that loosen stools and increase bowel movements. But now its use is restricted due to its carcinogenic activity.
Complete Step by step answer:
- The reaction between phenol and phthalic anhydride in the presence of sulphuric acid is a well- known reaction for the production of phenolphthalein.
- Sulphuric acid in this reaction acts as a dehydrating catalytic agent. Other agents, which can be used in place of sulfuric acid include anhydrous zinc chloride, anhydrous tin chloride, anhydrous aromatic sulfonic acids.
- Phenolphthalein is renowned as the indicator dye. Phenolphthalein can be synthesized by the condensation of phthalic anhydride with two equivalents of phenol under acidic condition, hence is named as Phenolphthalein by Adolf von Baeyer.

Note: Let us now see the various uses of phenolphthalein indicator:
(i) Use of pH indicator: It is most commonly used as an indicator in acid- base titrations. It also serves as a component or universal indicator when combined with methyl red, bromothymol blue, and thymol blue. Phenolphthalein acquires four different colors in different states of an aqueous solution. It is naturally colorless and remains colorless in acidic pH but turns pink in alkaline aqueous solutions.
(ii) Concrete carbonation: Concrete has naturally high pH due to the calcium hydroxide forms when Portland cement reacts with water. When a 1% solution of phenolphthalein is added to normal concrete, it turns pink, however, it remains colorless if the concrete has undergone carbonation.
(iii) Education: It shows a change of color from colorless to pink and is used in chemistry classes for the study of reaction kinetics.
(iv) Entertainment: It is also used as a component of disappearing inks or disappearing dyes on the Hollywood Barbie hair.
(v) Medical uses: It has been used as a laxative for a century. A laxative is the substance that loosen stools and increase bowel movements. But now its use is restricted due to its carcinogenic activity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




