
The type of bonds present in $CuS{O_4}.5{H_2}O$ are_____________.
A. electrovalent, covalent and coordinate
B. electrovalent and covalent
C. electrovalent and coordinate
D. covalent and coordinate
Answer
578.7k+ views
Hint: $CuS{O_4}.5{H_2}O$ is formed by two compounds Copper sulphate and water and also known blue vitriol. Copper sulphate has two ions copper ion and sulphate ion. Find the type of bond used to connect these two ions. And sulphate is formed by 1 Sulphur atom and 4 oxygen atoms. Determine the bond to form sulphate from sulphur and oxygen. Now to combine copper sulphate and water find what type of bond is used.
Complete step by step answer:
We are given to find the type of bond present in $CuS{O_4}.5{H_2}O$
$CuS{O_4}.5{H_2}O$ is known as Copper sulphate pentahydrate.
First to make Copper Sulphate $CuS{O_4}$ we need two ions Copper ion $C{u^{2 + }}$ and Sulphate ion$S{O_4}^{2 - }$
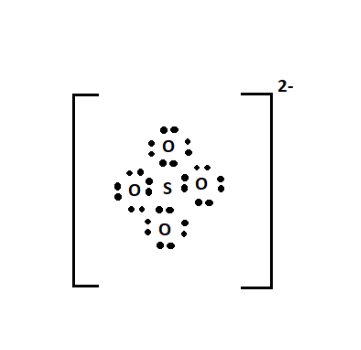
Sulphate ion is formed using 1 Sulphur atom and 4 oxygen atoms.
The sulphur is less electronegative compared to oxygen. So the middle atom in its molecular structure will be sulphur and 4 oxygen atoms are accommodated around it.
Oxygen and Sulphur atoms both have 6 valence electrons. So each sulphur and oxygen atom needs 2 more electrons to become stable and have an octet configuration.
So there must be 8 bonding electrons in sulphate. But the sulphur has only 6 electrons and 2 extra electrons come from two oxygen atoms’ half empty orbitals and shared between both of them. So the bond is a covalent bond.
Copper ion and sulphate ion combine to form copper sulphate by an ionic bond. An ionic bond is also known as an electrovalent bond.
$C{u^{2 + }} + S{O_4}^{2 - } \to CuS{O_4}$
Water is formed by covalent bonds between hydrogen and oxygen.
Copper sulphate and water combine to form copper sulphate pentahydrate complex by coordinating covalent bonds between Copper and water molecules. The electrons come only from water molecules and shared between copper and water.
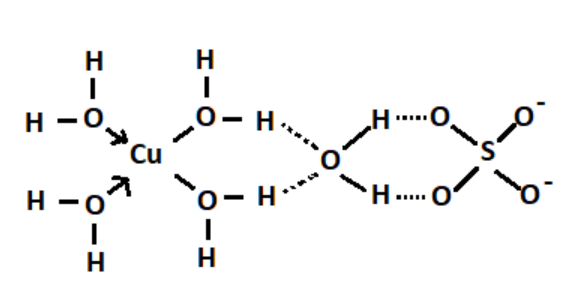
Therefore, electrovalent, covalent and coordinate covalent bonds are present in $CuS{O_4}.5{H_2}O$
Hence we can conclude that option A is correct.
Note:
Do not confuse between covalent bond and coordinate covalent bond. These both bonds happen between two non-metals but the difference is, in covalent bonds the electrons are being shared from both the atoms and in coordinate covalent bonds the shared pair of electrons comes from only one atom or one compound.
Complete step by step answer:
We are given to find the type of bond present in $CuS{O_4}.5{H_2}O$
$CuS{O_4}.5{H_2}O$ is known as Copper sulphate pentahydrate.
First to make Copper Sulphate $CuS{O_4}$ we need two ions Copper ion $C{u^{2 + }}$ and Sulphate ion$S{O_4}^{2 - }$
Sulphate ion is formed using 1 Sulphur atom and 4 oxygen atoms.
The sulphur is less electronegative compared to oxygen. So the middle atom in its molecular structure will be sulphur and 4 oxygen atoms are accommodated around it.
Oxygen and Sulphur atoms both have 6 valence electrons. So each sulphur and oxygen atom needs 2 more electrons to become stable and have an octet configuration.
So there must be 8 bonding electrons in sulphate. But the sulphur has only 6 electrons and 2 extra electrons come from two oxygen atoms’ half empty orbitals and shared between both of them. So the bond is a covalent bond.

Copper ion and sulphate ion combine to form copper sulphate by an ionic bond. An ionic bond is also known as an electrovalent bond.
$C{u^{2 + }} + S{O_4}^{2 - } \to CuS{O_4}$
Water is formed by covalent bonds between hydrogen and oxygen.
Copper sulphate and water combine to form copper sulphate pentahydrate complex by coordinating covalent bonds between Copper and water molecules. The electrons come only from water molecules and shared between copper and water.

Therefore, electrovalent, covalent and coordinate covalent bonds are present in $CuS{O_4}.5{H_2}O$
Hence we can conclude that option A is correct.
Note:
Do not confuse between covalent bond and coordinate covalent bond. These both bonds happen between two non-metals but the difference is, in covalent bonds the electrons are being shared from both the atoms and in coordinate covalent bonds the shared pair of electrons comes from only one atom or one compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




