
The type of overlapping in Br-F bond in $ Br{{F}_{3}} $ molecule is:
(A) $ s{{p}^{3}}-p $
(B) $ s{{p}^{2}}-p $
(C) $ s{{p}^{3}}d-p $
(D) $ s{{p}^{3}}{{d}^{2}}-p $
Answer
552.3k+ views
Hint: Hybridization is the concept of mixing of two atomic orbitals with the same energy levels to give a new type of degenerate orbitals. $ Br{{F}_{3}} $ has a Bent T shaped structure with Br as a central atom bonded with three F atoms (three bond pairs) and two lone pairs. So, the hybridization would be $ s {{p} ^ {3}} d $ .
Complete Step By Step Solution
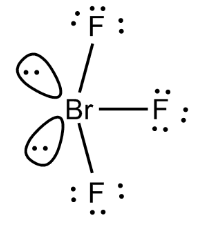
To determine the hybridization of bromine trifluoride we will first take the bromine atom which is the central atom and look at its electron configuration. It is represented as-
$ 1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}4{{p}^{5}} $ . But to form bonds with the fluorine atom some electrons in Bromine are shifted to 4d-orbitals. Also due to higher oxidative capacity of fluorine, it forces bromine atoms to promote electrons to the d- level. Now, d-orbitals are used for hybridization. $ Br{{F}_{3}} $ will consist of seven electrons in its outermost shell. After the bond formation, it will further have 2 lone pairs and 3 Br—F covalent bonds. As the hybridization value is equal to 5 it gives rise to $ s{{p}^{3}}d $ hybrid orbitals. A bond with p orbital of Fluorine is formed.
Hence, option-(C) is correct.
$ Br{{F}_{3}} $ has T-shaped or Trigonal Bipyramidal molecular geometry with a bond angle of $ 86\cdot 2{}^\circ $ .
Additional Information
Types of hybridization: The sp hybridization is observed when one s and one p orbital in the same main shell of an atom mix to form two new equivalent orbitals. The new orbitals formed are called sp $ 180{} ^ \circ $ hybridized orbitals. It forms linear molecules with an angle of $ 180{} ^ \circ $ .
$ s {{p} ^ {2}} $ hybridization is observed when one s and two p orbitals of the same shell of an atom mix to form 3 equivalent orbitals. The new orbitals formed are called $ s {{p} ^ {2}} $ hybrid orbitals. It is also called trigonal hybridization. Atomic orbitals with equal energies undergo hybridization.
Note
The central atom bromine uses the d-orbitals for hybridization. Lone pairs take part in hybridization. The electrons $ s{{p}^{3}} $ hybridized species are held farther from the nucleus than those in $ s{{p}^{2}} $ ( $ 33% $ s-character) and sp ( $ 50% $ s-character) hybridized species. The closer the electrons are to the nucleus, the more stable they are.
Complete Step By Step Solution

To determine the hybridization of bromine trifluoride we will first take the bromine atom which is the central atom and look at its electron configuration. It is represented as-
$ 1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}4{{p}^{5}} $ . But to form bonds with the fluorine atom some electrons in Bromine are shifted to 4d-orbitals. Also due to higher oxidative capacity of fluorine, it forces bromine atoms to promote electrons to the d- level. Now, d-orbitals are used for hybridization. $ Br{{F}_{3}} $ will consist of seven electrons in its outermost shell. After the bond formation, it will further have 2 lone pairs and 3 Br—F covalent bonds. As the hybridization value is equal to 5 it gives rise to $ s{{p}^{3}}d $ hybrid orbitals. A bond with p orbital of Fluorine is formed.
Hence, option-(C) is correct.
$ Br{{F}_{3}} $ has T-shaped or Trigonal Bipyramidal molecular geometry with a bond angle of $ 86\cdot 2{}^\circ $ .
Additional Information
Types of hybridization: The sp hybridization is observed when one s and one p orbital in the same main shell of an atom mix to form two new equivalent orbitals. The new orbitals formed are called sp $ 180{} ^ \circ $ hybridized orbitals. It forms linear molecules with an angle of $ 180{} ^ \circ $ .
$ s {{p} ^ {2}} $ hybridization is observed when one s and two p orbitals of the same shell of an atom mix to form 3 equivalent orbitals. The new orbitals formed are called $ s {{p} ^ {2}} $ hybrid orbitals. It is also called trigonal hybridization. Atomic orbitals with equal energies undergo hybridization.
Note
The central atom bromine uses the d-orbitals for hybridization. Lone pairs take part in hybridization. The electrons $ s{{p}^{3}} $ hybridized species are held farther from the nucleus than those in $ s{{p}^{2}} $ ( $ 33% $ s-character) and sp ( $ 50% $ s-character) hybridized species. The closer the electrons are to the nucleus, the more stable they are.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




