
The types of bonds present in sulphuric anhydride are
A. ${{3\sigma }}$ and ${{3}}{{{p}}_{{\pi }}} - {{{d}}_{{\pi }}}$
B. ${{3\sigma }}$, ${\text{1}}\,{{{p}}_{{\pi }}} - {{\text{p}}_{{\pi }}}$ and ${\text{2}}{{\text{p}}_{{\pi }}} - {{\text{d}}_{{\pi }}}$
C. ${{2\sigma }}$ and ${{3}}{{{p}}_{{\pi }}} - {{\text{d}}_{{\pi }}}$
D. ${{2\sigma }}$ and $2p_{\pi}- d_{\pi}$
Answer
559.8k+ views
Hint: To determine the answer we should know about hybridization. First we will draw the Lewis structure and by using VSEPR theory we will determine the hybridization to determine the number of sigma and pi bonds. We will also draw the hybridization to determine the orbitals forming sigma and pi bonds.
Complete step-by-step answer:
The sulphur trioxide is known as sulphuric anhydride. The chemical formula of sulphuric anhydride is ${\text{S}}{{\text{O}}_{\text{3}}}$.
We will write the Lewis structure for this we will write the basic structure. Then we will arrange all the valance electrons around each atom to complete the octet. According to valence shell electron pair repulsion theory, the electron pairs get arranged around the central to minimize the repulsion giving a specific geometry. The hybridization and geometry is decided based on the number of sigma and lone pairs around the central atom.
The Lewis structure of ${\text{S}}{{\text{O}}_{\text{3}}}$is as follows:
Total valence electrons are as follows:
$ = \,\left( {6 \times 1} \right) + \left( {6 \times 3} \right)$
$ = \,24$
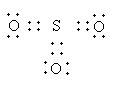
The sigma electron pairs around the sulphur atom is three. So, the hybridization and geometry of ${\text{S}}{{\text{O}}_{\text{3}}}$is ${\text{s}}{{\text{p}}^{\text{2}}}$ and trigonal planar.
So, sulphuric anhydride has three sigma and three pi bonds.
The hybridization in ${\text{S}}{{\text{O}}_{\text{3}}}$ is shown as follows:
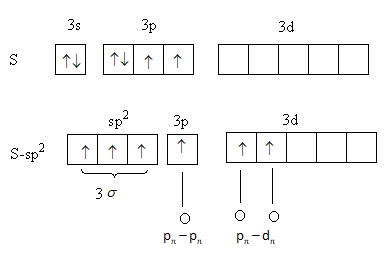
The valence configuration of sulphur is, ${\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{4}}}{\text{3d}}$ .
The valence configuration of oxygen anhydride is, ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{4}}}$ .
One s and two p-orbitals of sulphur combine to form three ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised orbitals. Two electrons one form s and one from p-orbital, transfer into d-orbital. Three ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised orbitals forms three sigma bonds with oxygen atoms. The next p-orbital forms one pi bond. The two d-orbital form two remaining two pi bonds.
So, the types of bonds present in sulphuric anhydride are ${{3\sigma }}$, ${\text{1}}\,{{\text{p}}_{{\pi }}} - {{{p}}_{{\pi }}}$ and ${\text{2}}{{{p}}_{{\pi }}} - {{\text{d}}_{{\pi }}}$.
Therefore, option (B) ${{3\sigma }}$, ${\text{1}}\,{{{p}}_{{\pi }}} - {{\text{p}}_{{\pi }}}$ and ${\text{2}}{{\text{p}}_{{\pi }}} - {{\text{d}}_{{\pi }}}$ is correct.
Note: Hybridised orbitals form sigma bonds only. So, the number of hybridised orbitals are equal to the number of sigma bonds. s-orbital always forms a sigma bond only. The pi bonds are formed by p and d-orbitals. Each atom donates an electron for both sigma and pi bond. Hydride orbits have the same energy.
Complete step-by-step answer:
The sulphur trioxide is known as sulphuric anhydride. The chemical formula of sulphuric anhydride is ${\text{S}}{{\text{O}}_{\text{3}}}$.
We will write the Lewis structure for this we will write the basic structure. Then we will arrange all the valance electrons around each atom to complete the octet. According to valence shell electron pair repulsion theory, the electron pairs get arranged around the central to minimize the repulsion giving a specific geometry. The hybridization and geometry is decided based on the number of sigma and lone pairs around the central atom.
The Lewis structure of ${\text{S}}{{\text{O}}_{\text{3}}}$is as follows:
Total valence electrons are as follows:
$ = \,\left( {6 \times 1} \right) + \left( {6 \times 3} \right)$
$ = \,24$

The sigma electron pairs around the sulphur atom is three. So, the hybridization and geometry of ${\text{S}}{{\text{O}}_{\text{3}}}$is ${\text{s}}{{\text{p}}^{\text{2}}}$ and trigonal planar.
So, sulphuric anhydride has three sigma and three pi bonds.
The hybridization in ${\text{S}}{{\text{O}}_{\text{3}}}$ is shown as follows:

The valence configuration of sulphur is, ${\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{4}}}{\text{3d}}$ .
The valence configuration of oxygen anhydride is, ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{4}}}$ .
One s and two p-orbitals of sulphur combine to form three ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised orbitals. Two electrons one form s and one from p-orbital, transfer into d-orbital. Three ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised orbitals forms three sigma bonds with oxygen atoms. The next p-orbital forms one pi bond. The two d-orbital form two remaining two pi bonds.
So, the types of bonds present in sulphuric anhydride are ${{3\sigma }}$, ${\text{1}}\,{{\text{p}}_{{\pi }}} - {{{p}}_{{\pi }}}$ and ${\text{2}}{{{p}}_{{\pi }}} - {{\text{d}}_{{\pi }}}$.
Therefore, option (B) ${{3\sigma }}$, ${\text{1}}\,{{{p}}_{{\pi }}} - {{\text{p}}_{{\pi }}}$ and ${\text{2}}{{\text{p}}_{{\pi }}} - {{\text{d}}_{{\pi }}}$ is correct.
Note: Hybridised orbitals form sigma bonds only. So, the number of hybridised orbitals are equal to the number of sigma bonds. s-orbital always forms a sigma bond only. The pi bonds are formed by p and d-orbitals. Each atom donates an electron for both sigma and pi bond. Hydride orbits have the same energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




