
The water equivalent of a calorimeter is 10g and it contains 50g of water at 15\[^0C\]. Some amount of ice, initially at −10\[^0C\] is dropped in it and half of the ice melts till equilibrium is reached. What was the initial amount of ice that was dropped (when specific heat of ice =0.5cal\[g{m^{ - 1}}^0{C^{ - 1}}\], specific heat of water =1.0cal\[g{m^{ - 1}}^0{C^{ - 1}}\] and latest heat of melting of ice =80 Cal\[g{m^{ - 1}}\])?
A.10 g
B.18 g
C.20 g
D.30 g
Answer
597k+ views
Hint: In this question we will learn about calorimeter, water equivalent, specific heat of ice and specific heat of water and the concepts behind these terms. These concepts will help us in solving this problem.
Complete answer:
Below here these concepts are discussed properly now we are going to take them one after another:
Calorimeter: It was first discovered by Antoine Lavoisier. He dubbed the apparatus as the calorimeter, based on the two Greek and Latin roots. A calorimeter is a mechanical assembly whose work is used for calorimetry, or the procedure of measuring the heat released or absorbed in a chemical reaction or physical changes and heat capacity. A simple calorimeter is made up of a thermometer connected to a metal vessel which is full of water hanging above a combustion chamber.
Specific heat of water and ice: The specific heat capacity is donated by symbol {Cp}. Specific heat is basically the amount of energy that should be added, in the form of heat, to unit mass of the item in order to cause a rise of one unit in its temperature. The SI unit coined for specific heat is joule per kelvin.
Water Equivalent: water equivalent of a target is equivalent to the amount of water necessary to suck-up the same amount of heat as that target does for one degree of rise in temperature. Therefore, the water equivalent of a body is the same as the product of its mass and its specific heat.
Water equivalent is mathematically formulated by mass x specific heat
Water equivalent is mathematically formulated by Heat capacity
$
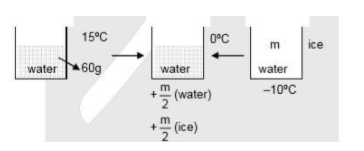
m \times \dfrac{1}{2} \times 10 + \dfrac{m}{2} \times 80 = 60 \times 1 \times 15 \\
m = \dfrac{{60 \times 15}}{{45}} = 20gm \\
$
Note:In this question we learned about water equivalent, specific heat of ice and water and abbot calorimeter. We learned these concepts and learned about their mathematical uses. We have applied all these concepts in these questions.
Complete answer:
Below here these concepts are discussed properly now we are going to take them one after another:
Calorimeter: It was first discovered by Antoine Lavoisier. He dubbed the apparatus as the calorimeter, based on the two Greek and Latin roots. A calorimeter is a mechanical assembly whose work is used for calorimetry, or the procedure of measuring the heat released or absorbed in a chemical reaction or physical changes and heat capacity. A simple calorimeter is made up of a thermometer connected to a metal vessel which is full of water hanging above a combustion chamber.
Specific heat of water and ice: The specific heat capacity is donated by symbol {Cp}. Specific heat is basically the amount of energy that should be added, in the form of heat, to unit mass of the item in order to cause a rise of one unit in its temperature. The SI unit coined for specific heat is joule per kelvin.
Water Equivalent: water equivalent of a target is equivalent to the amount of water necessary to suck-up the same amount of heat as that target does for one degree of rise in temperature. Therefore, the water equivalent of a body is the same as the product of its mass and its specific heat.
Water equivalent is mathematically formulated by mass x specific heat
Water equivalent is mathematically formulated by Heat capacity
$
m \times \dfrac{1}{2} \times 10 + \dfrac{m}{2} \times 80 = 60 \times 1 \times 15 \\
m = \dfrac{{60 \times 15}}{{45}} = 20gm \\
$

Note:In this question we learned about water equivalent, specific heat of ice and water and abbot calorimeter. We learned these concepts and learned about their mathematical uses. We have applied all these concepts in these questions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




