
The yield of ketone when a secondary alcohol is oxidized in presence of excess oxidizing agent is _______ than the yield of aldehyde when a primary alcohol is oxidized in presence of excess oxidizing agent.
(A) More
(B) Less
(C) Same
(D) None of these
Answer
525k+ views
Hint :In a secondary alcohol, the alpha carbon bonded with the alcohol group is bonded with only one hydrogen. Hence, on oxidation, the hydrogen is removed and ketone is formed. Now, there are no other products possible other than ketone. While for a primary alcohol, two hydrogens are bonded to primary carbon, and after oxidation to aldehyde, it still has a chance to carry out further oxidation and thus aldehyde will not be the only preferred product present.
Complete Step By Step Answer:
The oxidation reaction can be defined as the addition of oxygen or the removal of hydrogen from a compound. As hydrogen is unstable in monoatomic state, in oxidation, hydrogen will be removed in pairs and a new bond will be made.
Now, let us consider the reaction of secondary alcohol in presence of oxidising agent, which is shown as
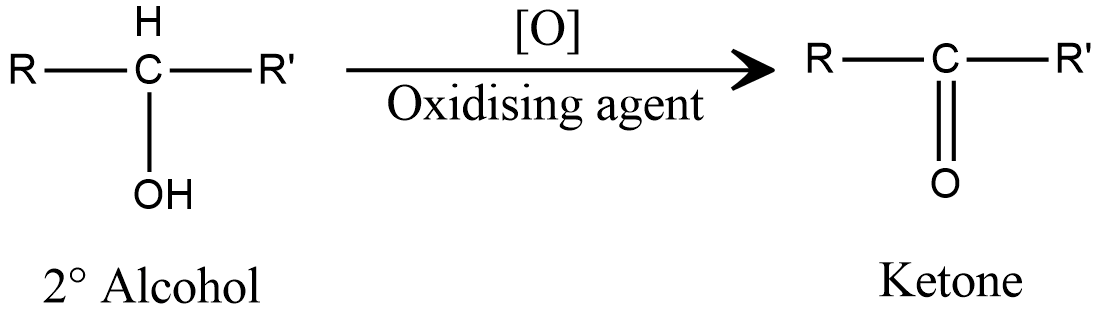
This is the final product obtained after oxidation will be ketone, and no other further oxidation is possible, as there is no hydrogen left on alpha carbon to eliminate.
Now, let us consider the reaction of primary alcohol in presence of excess oxidising agent, which is shown as
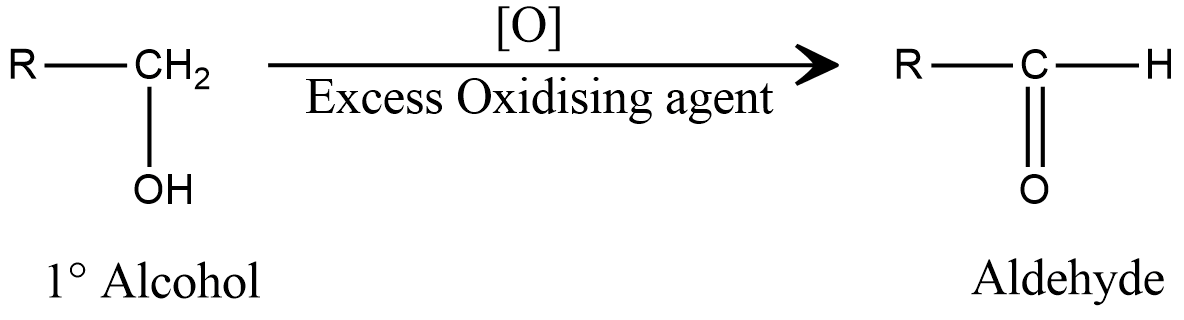
Now, here the alpha carbon still contains a hydrogen atom and excess oxidising agent to carry out the oxidation reaction, leading to the formation of carboxylic acid
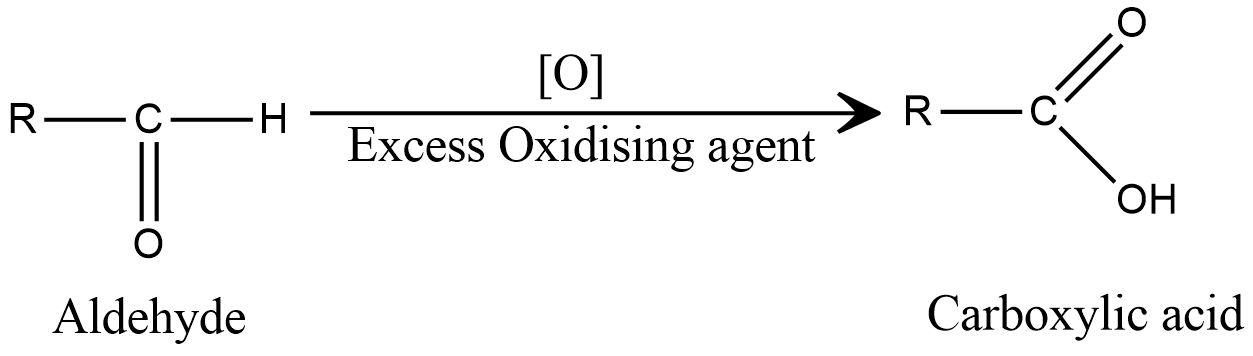
Hence, due to the excess oxidising agent, the reaction does not stop at aldehyde, and hence not much quantity of aldehyde is obtained.
However, in production of ketone, only ketone is produced and hence the quantity of the product will be higher.
Hence, the correct answer is Option (A).
Note :
We can see from the above reactions that aldehyde is not obtained in the oxidation of primary alcohol, rather it gets further converted to carboxylic acid. Hence, this method is used in the production of carboxylic acid. However, if we are required to produce aldehyde as the final product, we can either take the oxidising agent in limited quantity, so that no catalyst is left to carry the reaction further after the production of aldehyde, or we can take a weak oxidising agent like pyridinium chloro-chromate, which is not capable to replace hydrogen with hydroxyl group, and hence, final product will be aldehyde.
Complete Step By Step Answer:
The oxidation reaction can be defined as the addition of oxygen or the removal of hydrogen from a compound. As hydrogen is unstable in monoatomic state, in oxidation, hydrogen will be removed in pairs and a new bond will be made.
Now, let us consider the reaction of secondary alcohol in presence of oxidising agent, which is shown as

This is the final product obtained after oxidation will be ketone, and no other further oxidation is possible, as there is no hydrogen left on alpha carbon to eliminate.
Now, let us consider the reaction of primary alcohol in presence of excess oxidising agent, which is shown as
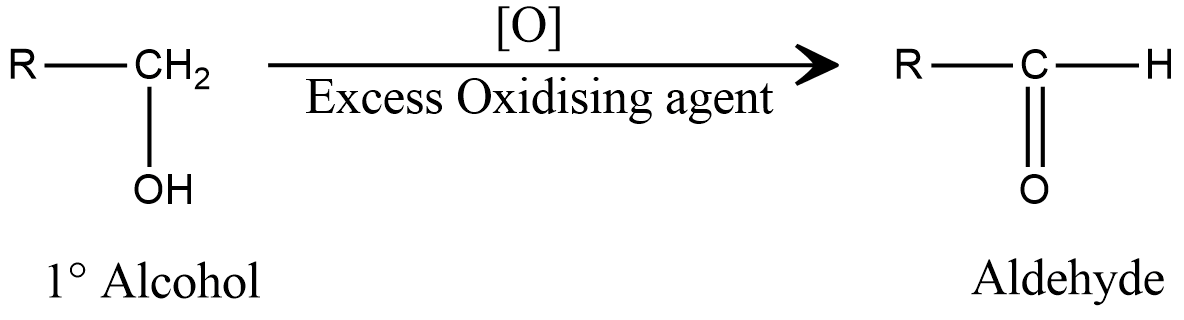
Now, here the alpha carbon still contains a hydrogen atom and excess oxidising agent to carry out the oxidation reaction, leading to the formation of carboxylic acid

Hence, due to the excess oxidising agent, the reaction does not stop at aldehyde, and hence not much quantity of aldehyde is obtained.
However, in production of ketone, only ketone is produced and hence the quantity of the product will be higher.
Hence, the correct answer is Option (A).
Note :
We can see from the above reactions that aldehyde is not obtained in the oxidation of primary alcohol, rather it gets further converted to carboxylic acid. Hence, this method is used in the production of carboxylic acid. However, if we are required to produce aldehyde as the final product, we can either take the oxidising agent in limited quantity, so that no catalyst is left to carry the reaction further after the production of aldehyde, or we can take a weak oxidising agent like pyridinium chloro-chromate, which is not capable to replace hydrogen with hydroxyl group, and hence, final product will be aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




