
Thermosetting polymer, Bakelite is formed by the reaction of phenol with:
(A) \[C{H_3}C{H_2}CHO\]
(B) \[C{H_3}CHO\]
(C) \[HCHO\]
(D) \[HCOOH\]
Answer
586.2k+ views
Hint:Thermosetting polymer is a cross-linked polymer which once heated gets hardened and can not be moulded again. This means that these polymers can’t be softened again on heating. Bakelite is a thermosetting plastic formed by condensation reaction of phenol and formaldehyde.
Complete step by step answer:
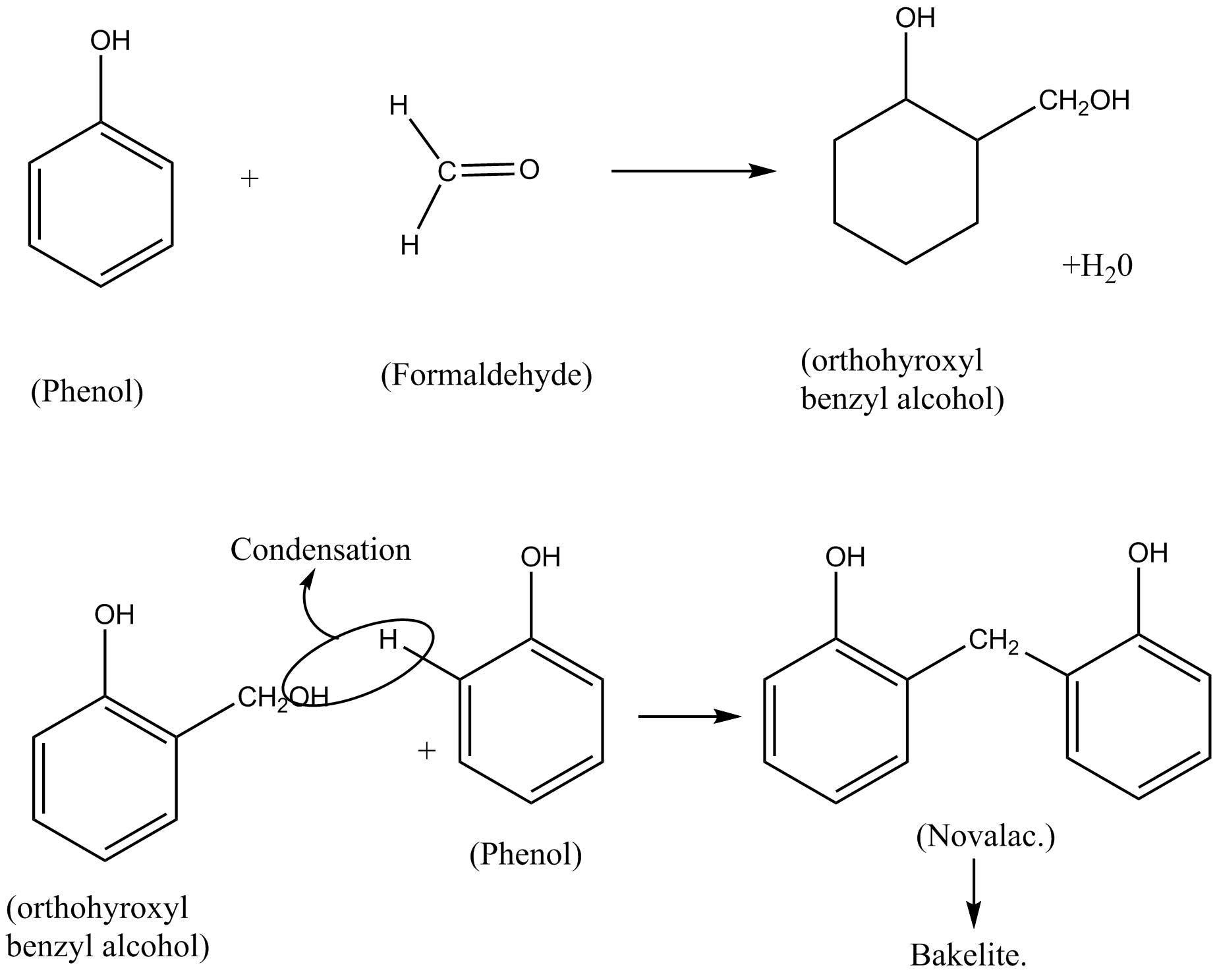
Bakelite is a thermosetting phenol formaldehyde resin. It is a polymer made up of two monomers that are phenol and formaldehyde\[\left( {HCHO} \right)\]. The reaction involves condensation of monomers in the presence of a catalyst.
The reaction of phenol and formaldehyde have three main steps:
Step I – Reaction of phenol and formaldehyde to give ortho and para hydroxy benzyl alcohol.
Step II – Ortho hydroxybenzyl alcohol on removal of water molecules gives Novalac.
Step III – Novolac forms Bakelite.
Hence, the correct option is (C) \[HCHO\].
Additional information: Bakelite was developed by Leo Baekeland in \[1907\]. It is the first synthetic plastic. It is used in radio, televisions, insulators etc because it has heat – resistant and insulating properties. It is used commonly in making kitchenware.
Bakelite is a smooth molding polymer, which gets molded very quickly. It is non – conductor of heat and electricity. Bakelite is a thermosetting polymer, so it retains its shape forever once molded. Handles of utensils are made up of bakelite so that the handles do not get deformed and do not harm the user.
Note:
The common catalysts used in production of bakelite are zinc chloride\[\left( {ZnC{l_2}} \right)\], hydrochloric acid \[\left( {HCl} \right)\], ammonia \[\left( {N{H_3}} \right)\] etc. It is heated at about \[{300^\circ }F\] or \[{150^\circ }C\] to make bakelite phenol and formaldehyde.
General formula of bakelite is \[{\left( {{C_6}{H_6}O \cdot C{H_2}O} \right)_n}\]
Complete step by step answer:
Bakelite is a thermosetting phenol formaldehyde resin. It is a polymer made up of two monomers that are phenol and formaldehyde\[\left( {HCHO} \right)\]. The reaction involves condensation of monomers in the presence of a catalyst.
The reaction of phenol and formaldehyde have three main steps:
Step I – Reaction of phenol and formaldehyde to give ortho and para hydroxy benzyl alcohol.
Step II – Ortho hydroxybenzyl alcohol on removal of water molecules gives Novalac.
Step III – Novolac forms Bakelite.

Hence, the correct option is (C) \[HCHO\].
Additional information: Bakelite was developed by Leo Baekeland in \[1907\]. It is the first synthetic plastic. It is used in radio, televisions, insulators etc because it has heat – resistant and insulating properties. It is used commonly in making kitchenware.
Bakelite is a smooth molding polymer, which gets molded very quickly. It is non – conductor of heat and electricity. Bakelite is a thermosetting polymer, so it retains its shape forever once molded. Handles of utensils are made up of bakelite so that the handles do not get deformed and do not harm the user.
Note:
The common catalysts used in production of bakelite are zinc chloride\[\left( {ZnC{l_2}} \right)\], hydrochloric acid \[\left( {HCl} \right)\], ammonia \[\left( {N{H_3}} \right)\] etc. It is heated at about \[{300^\circ }F\] or \[{150^\circ }C\] to make bakelite phenol and formaldehyde.
General formula of bakelite is \[{\left( {{C_6}{H_6}O \cdot C{H_2}O} \right)_n}\]
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




