
Total number of methyl group present in product A is:

Answer
588k+ views
Hint: Methyl group is that group which contains a single carbon atom attached with three hydrogen atoms. Given compound is an ester. Esters are derivatives of organic acids. The $ - OH$ group of acids is replaced by $ - OR$ group in esters.
Complete step by step answer:
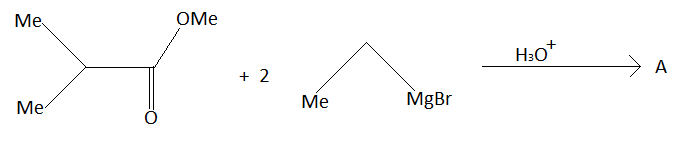
In this question given reactant is,
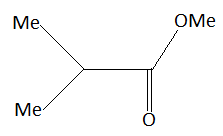
This is an ester. Esters are carboxylic acid derivatives. When the $ - OH$ group of carboxylic acid is replaced by $ - OR$, esters are formed. In this question ester reacts with $C{H_3}C{H_2}MgBr$. Esters react with $C{H_3}C{H_2}MgBr$ to form tertiary alcohols. Tertiary alcohols are represented by ${3^ \circ } - $alcohols. Tertiary alcohols are those alcohols in which the carbon atom to which alcohol is attached is surrounded with other three carbon atoms.
In this reaction when given ester will react with $C{H_3}C{H_2}MgBr$ than $ - OMe$ group of ester will be replaced with alkyl group of $C{H_3}C{H_2}MgBr$ and the oxygen that is bounded by a double bond with carbon atom will be reduced to alcohol. Given reactant can also be written as:
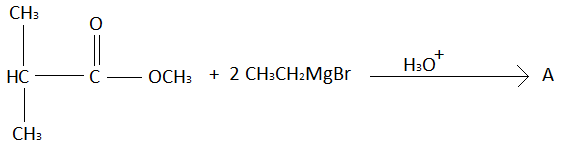
As explained above the product of this reaction will be,
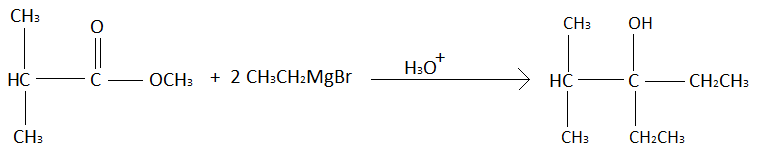
We have to count the number of methyl groups in the product obtained. Methyl group an alkyl group in which there is one carbon atom bonded with three hydrogen atoms. In the product formed there are a total four methyl groups.
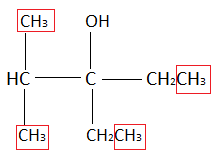
All the groups that are present in the red square in the above question represents the alkyl group. So, there are a total four alkyl groups in A (product formed from the given reaction).
Note:
A Wurtz fittig reaction is also an organic reaction. In this reaction aryl halides react with alkyl halide and sodium metal in the presence of dry ether to give substituted aromatic compounds. This reaction is best to form asymmetrical products.
Complete step by step answer:
In this question given reactant is,

This is an ester. Esters are carboxylic acid derivatives. When the $ - OH$ group of carboxylic acid is replaced by $ - OR$, esters are formed. In this question ester reacts with $C{H_3}C{H_2}MgBr$. Esters react with $C{H_3}C{H_2}MgBr$ to form tertiary alcohols. Tertiary alcohols are represented by ${3^ \circ } - $alcohols. Tertiary alcohols are those alcohols in which the carbon atom to which alcohol is attached is surrounded with other three carbon atoms.
In this reaction when given ester will react with $C{H_3}C{H_2}MgBr$ than $ - OMe$ group of ester will be replaced with alkyl group of $C{H_3}C{H_2}MgBr$ and the oxygen that is bounded by a double bond with carbon atom will be reduced to alcohol. Given reactant can also be written as:
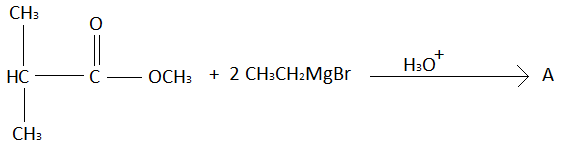
As explained above the product of this reaction will be,
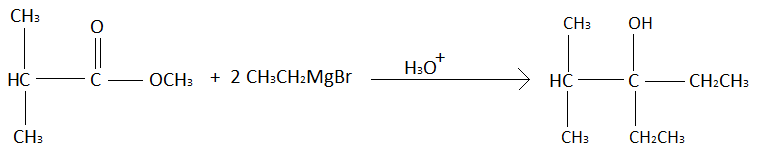
We have to count the number of methyl groups in the product obtained. Methyl group an alkyl group in which there is one carbon atom bonded with three hydrogen atoms. In the product formed there are a total four methyl groups.

All the groups that are present in the red square in the above question represents the alkyl group. So, there are a total four alkyl groups in A (product formed from the given reaction).
Note:
A Wurtz fittig reaction is also an organic reaction. In this reaction aryl halides react with alkyl halide and sodium metal in the presence of dry ether to give substituted aromatic compounds. This reaction is best to form asymmetrical products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




