
What is the total number of nodes present in the atomic orbitals that are occupied by electrons in the atom of Zn?
A.11
B.15
C.16
D.25
Answer
583.8k+ views
Hint:We know that l represents the orbital angular momentum quantum number and n represents principal quantum number. We are also familiar with two types of notes, angular and radial notes. The total notes of an orbital are the sum of angular and radial nodes and are given by the formula in terms of n and l quantum numbers.
Complete step-by-step answer:
There are two types of nodes present in the atomic orbitals, these are angular and radial nodes. Angular nodes are typically flat planes at fixed angles whereas radial nodes are spheres at fixed radius that occur as the principal quantum number increases. Angular nodes are determined by l quantum number and radial nodes are determined by the formula n – l – 1, where n is the principal quantum number and l is the azimuthal quantum number.
The nodal plane or angular node is a plane at which the probability of finding an electron is zero. Same is the case with radial nodes. The subshells in an orbital are denoted as s, p, d, f for which the azimuthal quantum number value or l value is 0, 1, 2, 3 respectively. These represent the angular nodes as well. The principal quantum number determines the value of the number of shells present in the atomic orbital.
So, now we know that the total number of nodes will be equal to the sum of angular nodes and radial nodes present in the atomic orbital. Let us add them and get the formula for the total number of nodes in an orbital.
Total number of nodes = angular nodes + radial nodes
Total number of nodes = l + n – l – 1 which is equal to n-1.
We know the electronic configuration of zinc in its ground state as \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}\] and its atomic number is 30. we can see that it has a total 7 atomic orbitals and we can calculate the number of nodes for each orbital.
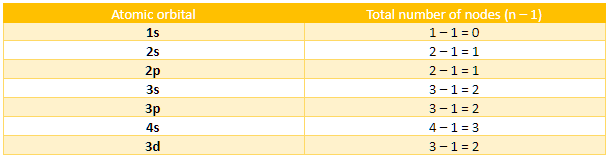
So, on adding them, we get the total number of nodes present in the atomic orbitals that are occupied by electrons in the atom of Zn as 11.
Hence, the correct option is (A).
Note: The number of radial nodes increases as the principal quantum number increases. But the number of angular nodes depends upon the shape of the orbital. The radial nodes calculate the distance from the nucleus whereas the angular node determines the direction as they depend on shape of the orbital such as s-orbital has spherical shape, p-orbital has dumbbell shape and d-orbital has double dumbbell shape.
Complete step-by-step answer:
There are two types of nodes present in the atomic orbitals, these are angular and radial nodes. Angular nodes are typically flat planes at fixed angles whereas radial nodes are spheres at fixed radius that occur as the principal quantum number increases. Angular nodes are determined by l quantum number and radial nodes are determined by the formula n – l – 1, where n is the principal quantum number and l is the azimuthal quantum number.
The nodal plane or angular node is a plane at which the probability of finding an electron is zero. Same is the case with radial nodes. The subshells in an orbital are denoted as s, p, d, f for which the azimuthal quantum number value or l value is 0, 1, 2, 3 respectively. These represent the angular nodes as well. The principal quantum number determines the value of the number of shells present in the atomic orbital.
So, now we know that the total number of nodes will be equal to the sum of angular nodes and radial nodes present in the atomic orbital. Let us add them and get the formula for the total number of nodes in an orbital.
Total number of nodes = angular nodes + radial nodes
Total number of nodes = l + n – l – 1 which is equal to n-1.
We know the electronic configuration of zinc in its ground state as \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}\] and its atomic number is 30. we can see that it has a total 7 atomic orbitals and we can calculate the number of nodes for each orbital.

So, on adding them, we get the total number of nodes present in the atomic orbitals that are occupied by electrons in the atom of Zn as 11.
Hence, the correct option is (A).
Note: The number of radial nodes increases as the principal quantum number increases. But the number of angular nodes depends upon the shape of the orbital. The radial nodes calculate the distance from the nucleus whereas the angular node determines the direction as they depend on shape of the orbital such as s-orbital has spherical shape, p-orbital has dumbbell shape and d-orbital has double dumbbell shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




