
Total number of possible structural isomers of ${{\text{C}}_5}{{\text{H}}_{{\text{11}}}}{\text{Br}}$ are:
A. 8
B. 7
C. 10
D. 9
Answer
581.4k+ views
Hint: Isomers are the species which have the same molecular formula but different structure. Consider the places where bromine can be displaced and different configurations of the main chain should be considered while answering the question.
Complete step by step answer:
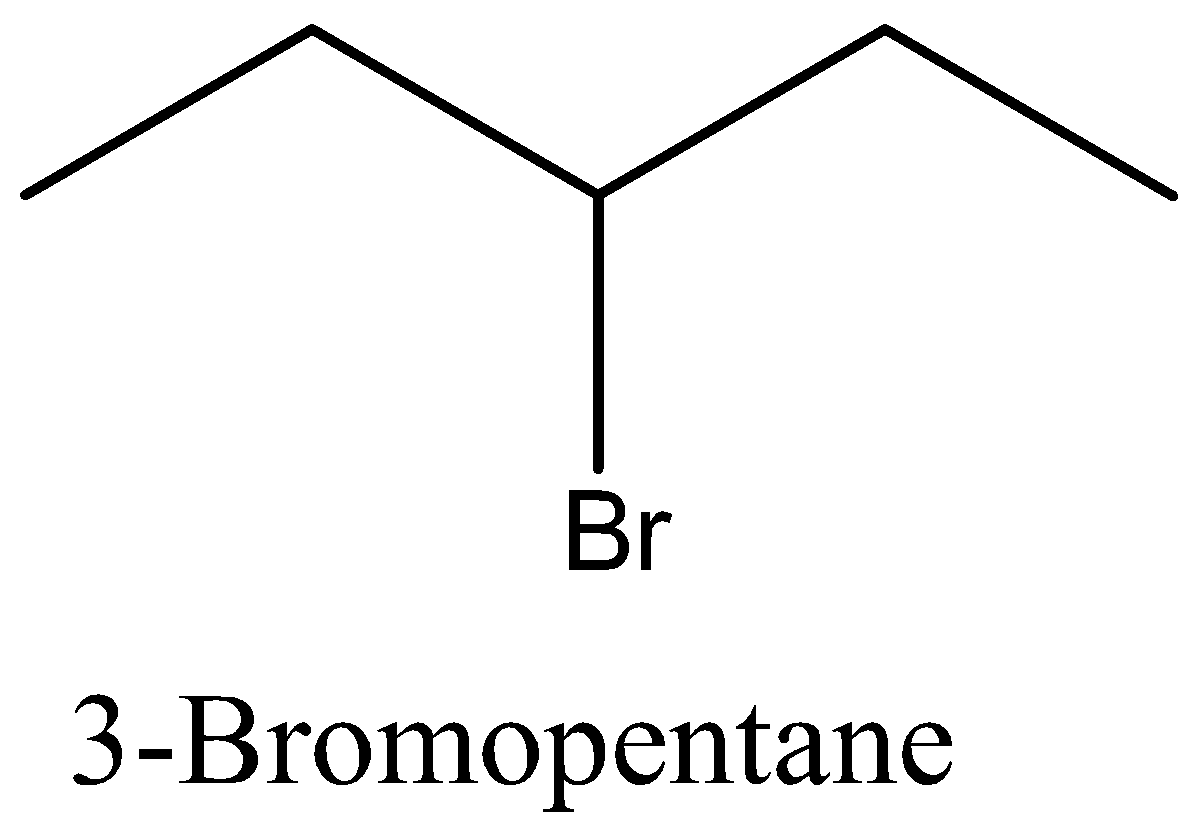
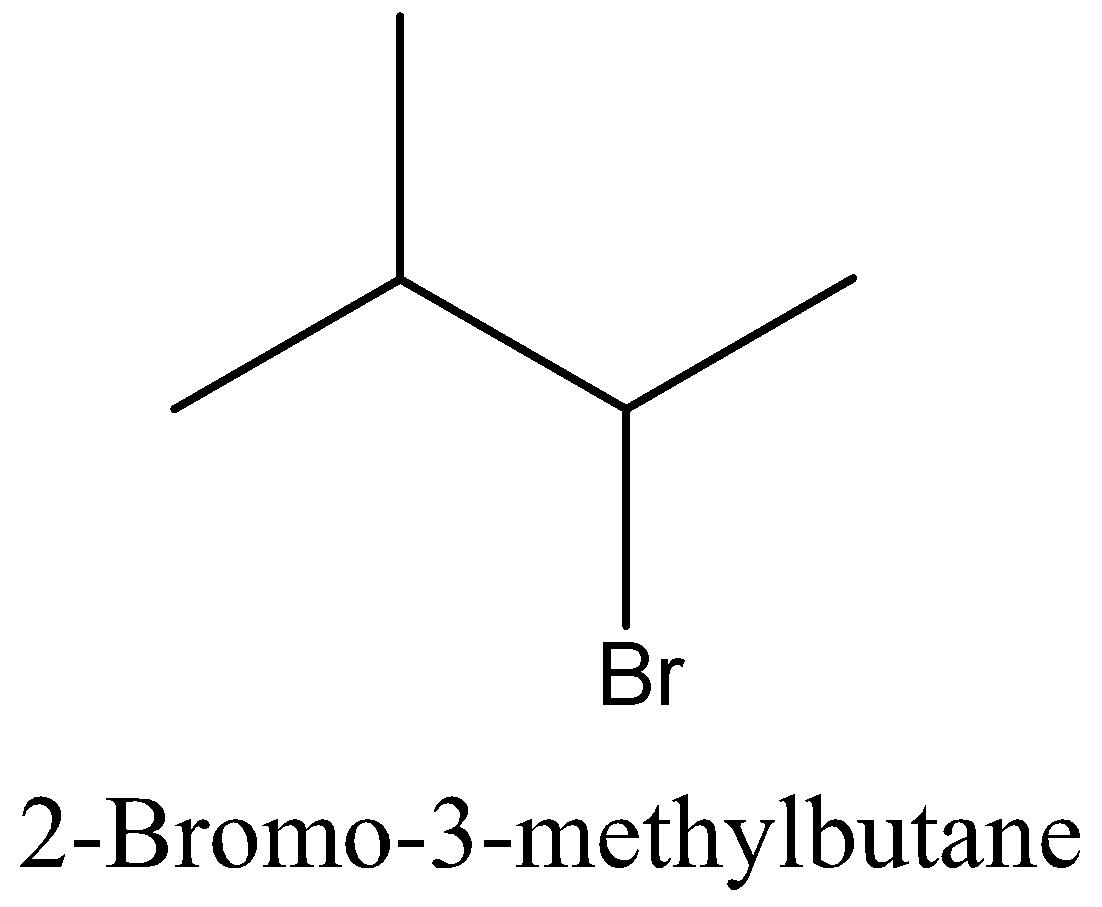
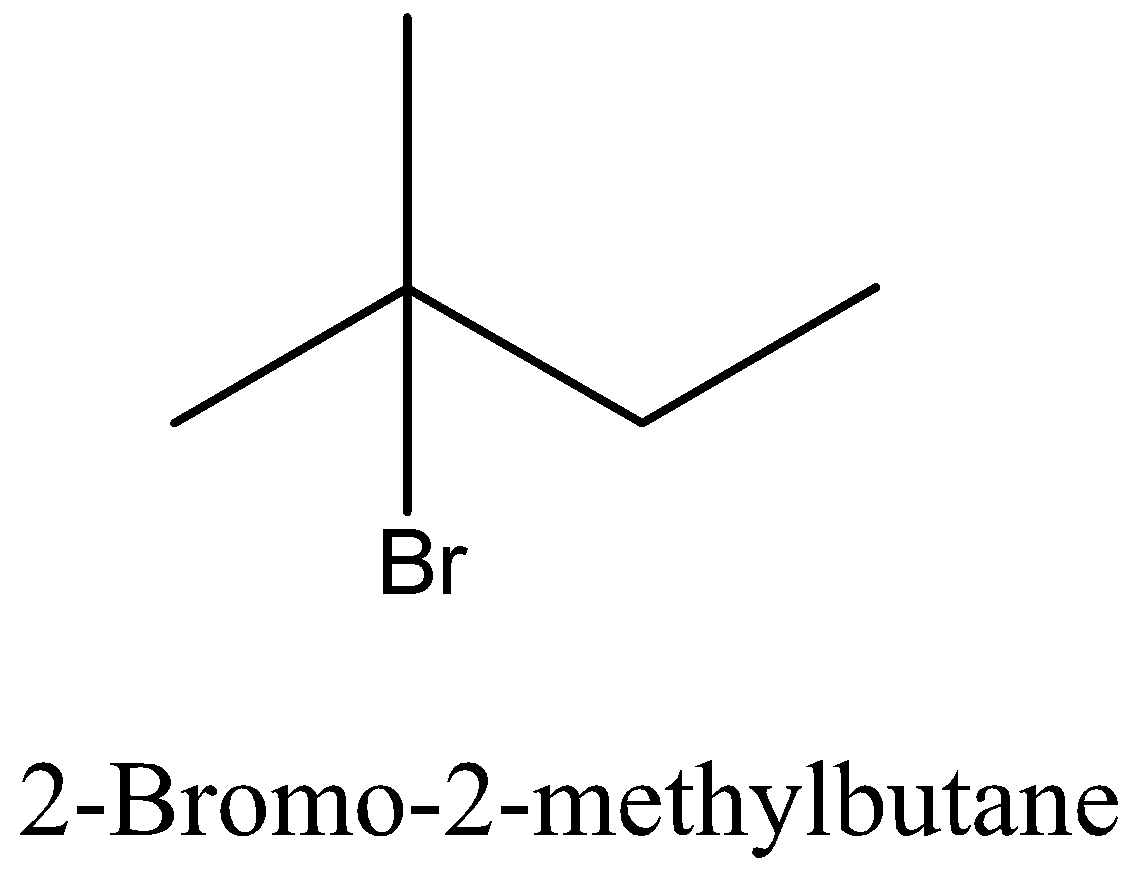
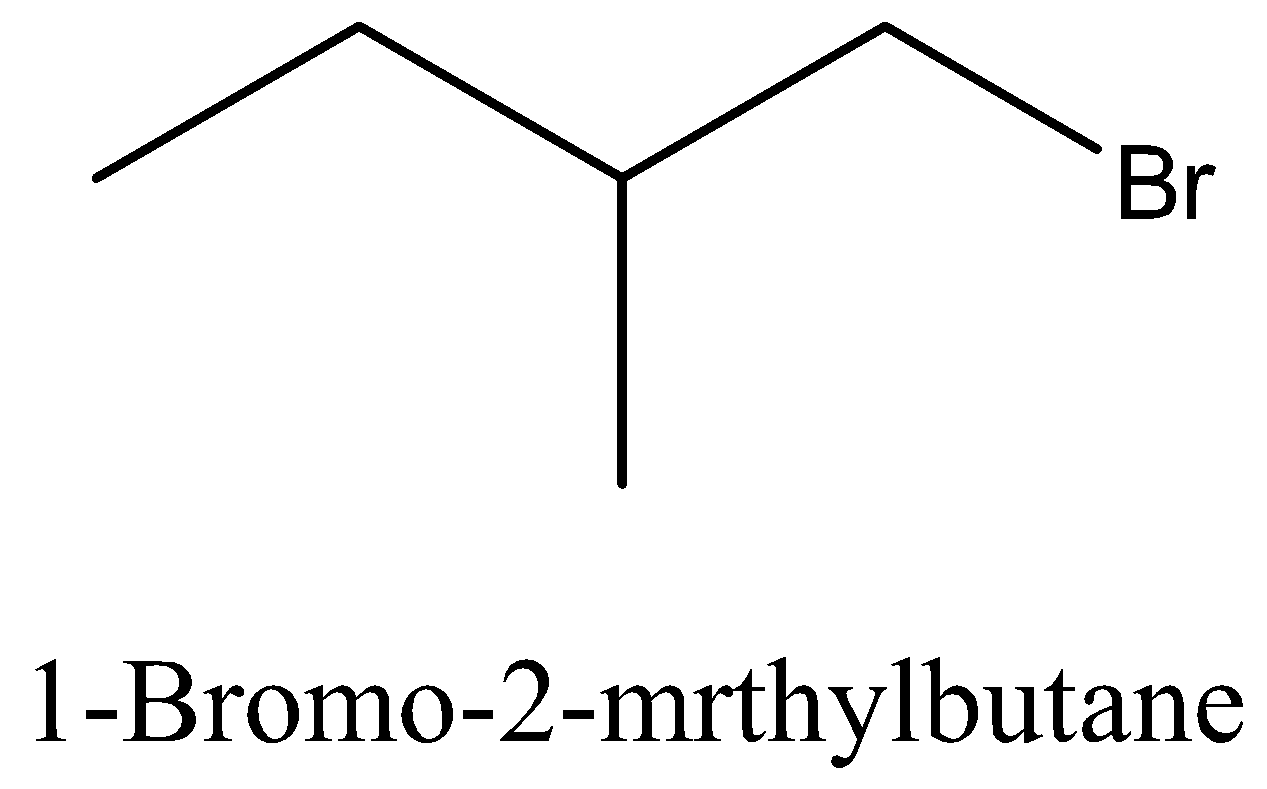
Let’s start with discussing the isomer for better understanding of the question. Isomers are species which have the same molecular formula but different structure. Let’s take an example of butane and isobutene, both of these are having the same molecular formula that is ${{\text{C}}_4}{{\text{H}}_{{\text{10}}}}$, but when it comes to the structure, butane is a straight chain and isobutene is having a branch of ${\text{C}}{{\text{H}}_3}$ at 2nd carbon.
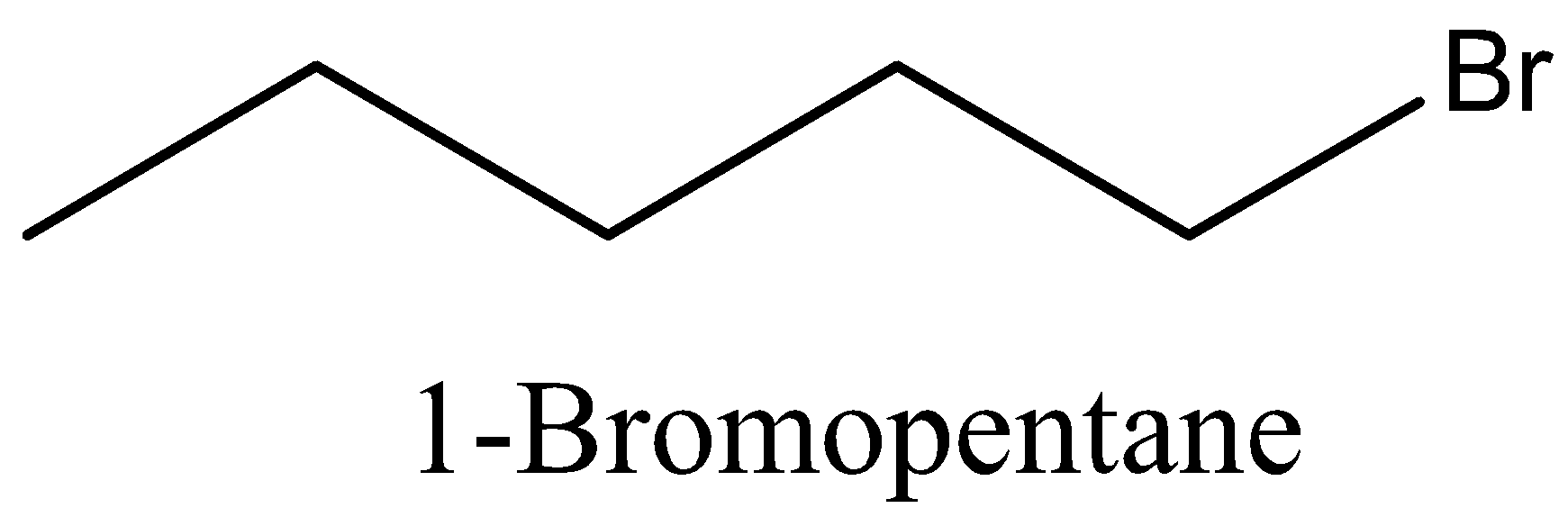
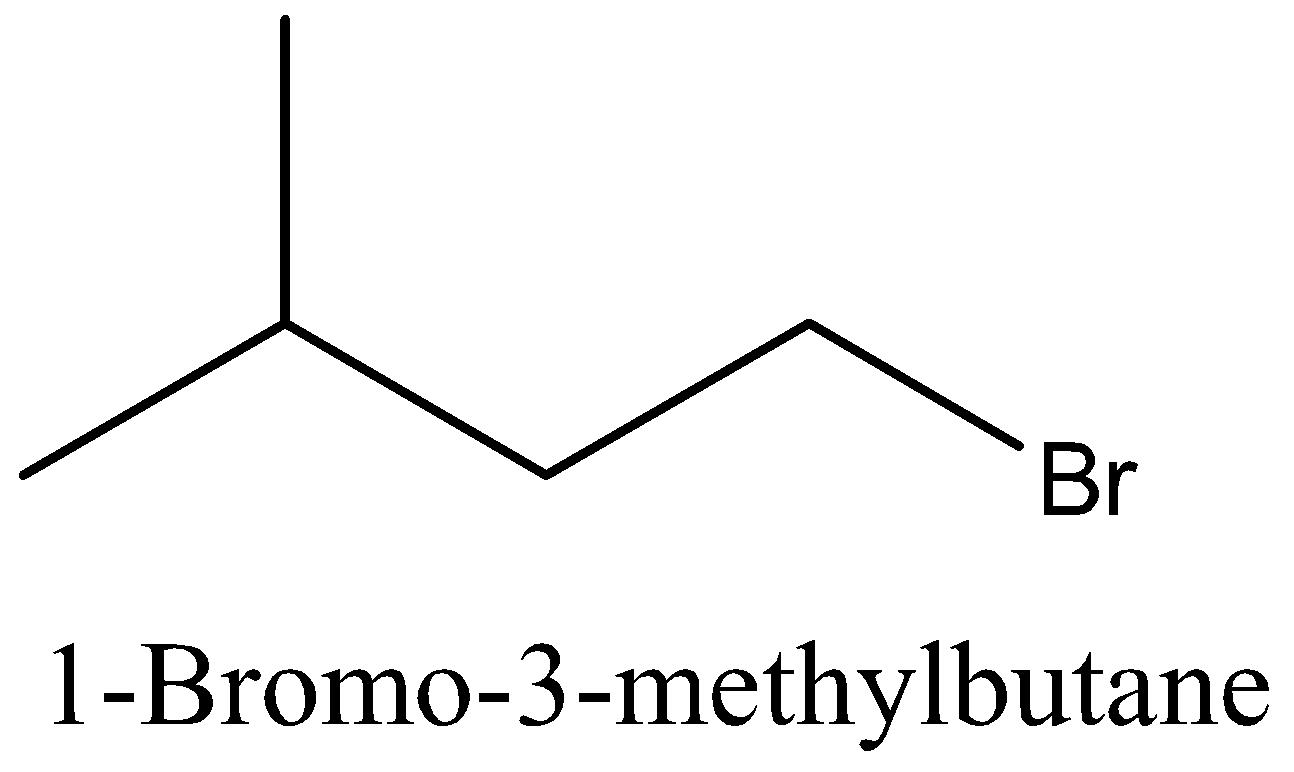
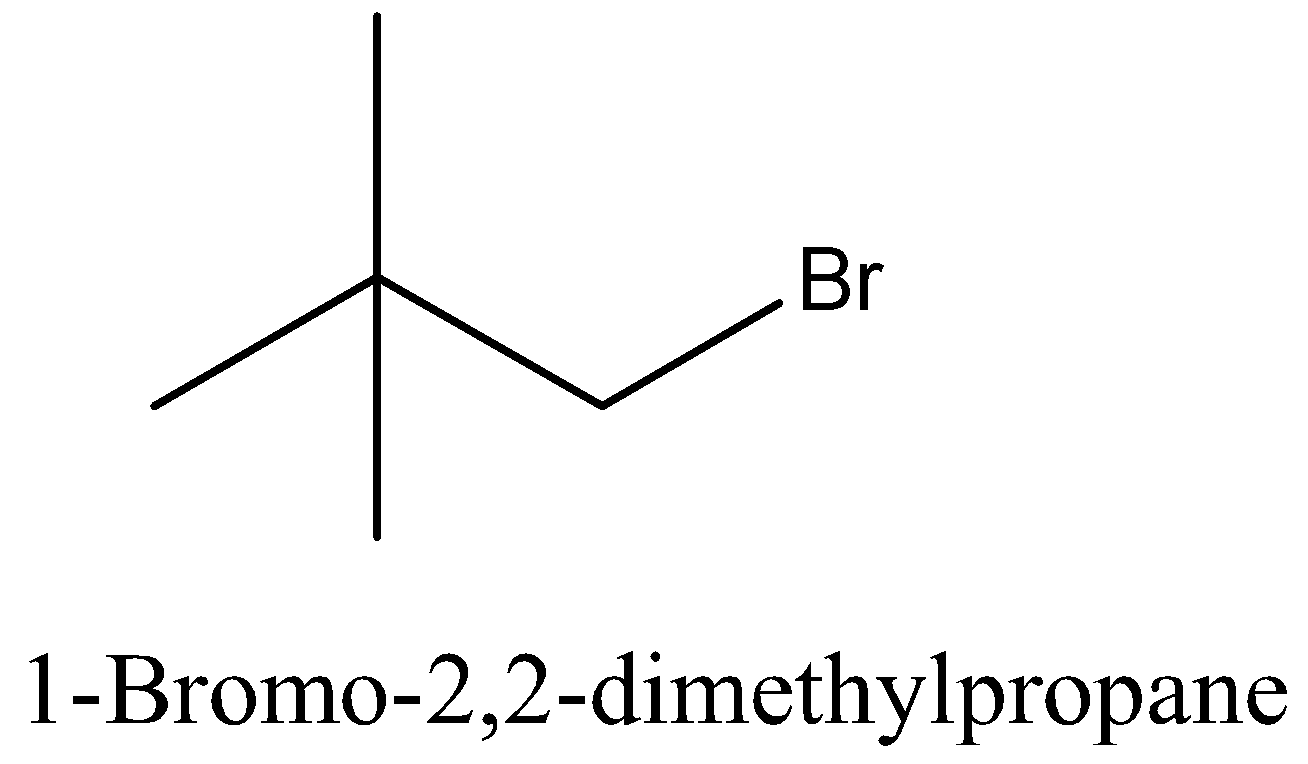
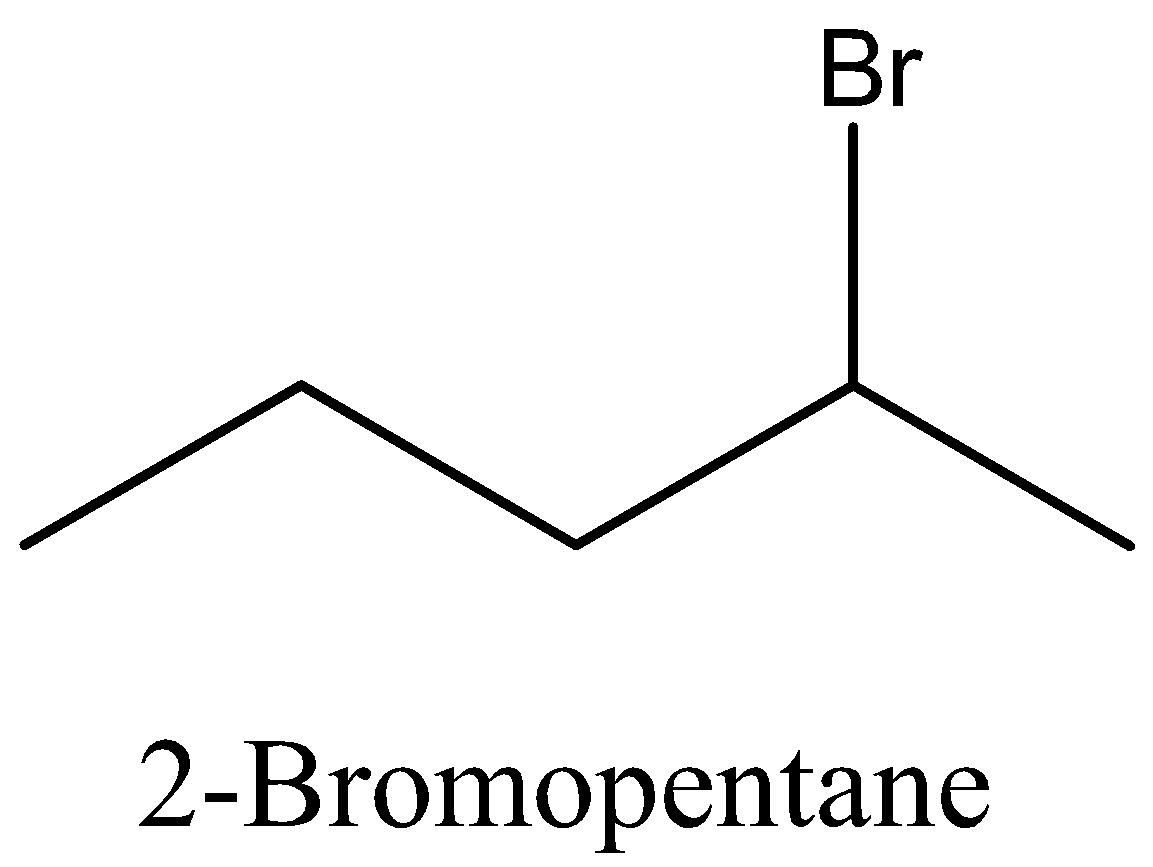
Coming back to our question we are given the molecular formula ${{\text{C}}_5}{{\text{H}}_{{\text{11}}}}{\text{Br}}$ and we have to find the total number of isomers. First we can place the Br in 3 different positions giving 3 different structures which are 1-bromopentane, 2-bromopentane, 3-bromopentane. After this we can change the position of methyl groups and get 5 more structure which are 1-bromo-2-methylbutane, 2-bromo-2-methylbutane, 2-bromo-3-methylbutane, 1-bromo-3-methylbutane, 1-bromo-2,2-dimethylpropane. These 8 structures are possible.
So, the correct answer is Option A.
Note:
We have to mind that Isomers are having similar molecular formulas and due to that they have weight, but isomers are having different molecular structure configuration and due to that they are having different physical properties but similar chemical properties.
Complete step by step answer:








Let’s start with discussing the isomer for better understanding of the question. Isomers are species which have the same molecular formula but different structure. Let’s take an example of butane and isobutene, both of these are having the same molecular formula that is ${{\text{C}}_4}{{\text{H}}_{{\text{10}}}}$, but when it comes to the structure, butane is a straight chain and isobutene is having a branch of ${\text{C}}{{\text{H}}_3}$ at 2nd carbon.
Coming back to our question we are given the molecular formula ${{\text{C}}_5}{{\text{H}}_{{\text{11}}}}{\text{Br}}$ and we have to find the total number of isomers. First we can place the Br in 3 different positions giving 3 different structures which are 1-bromopentane, 2-bromopentane, 3-bromopentane. After this we can change the position of methyl groups and get 5 more structure which are 1-bromo-2-methylbutane, 2-bromo-2-methylbutane, 2-bromo-3-methylbutane, 1-bromo-3-methylbutane, 1-bromo-2,2-dimethylpropane. These 8 structures are possible.
So, the correct answer is Option A.
Note:
We have to mind that Isomers are having similar molecular formulas and due to that they have weight, but isomers are having different molecular structure configuration and due to that they are having different physical properties but similar chemical properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




