
How many tripeptide bonds can be formed using a combination of three amino acids?
(i)- Glycine (ii)- Alanine (iii)- Phenylalanine
(a) 5
(b) 4
(c) 6
(d) 3
Answer
582k+ views
Hint: A peptide bond is a bond formed between the amine group of one $\alpha -\text{amino acid}$ and the carboxylic acid group of either with the same $\alpha -\text{amino acid}$ or a different $\alpha -\text{amino acid}$. Tripeptide bond means the molecule has three peptide bonds.
Complete step by step answer:
We know that an $\alpha -\text{amino acid}$ joins to another or the same $\alpha -\text{amino acid}$ by a bond called a peptide bond. $\alpha -\text{amino acid}$have two groups at its terminal carbon atoms i.e., an amine group and a carboxylic acid group. The amine group of one $\alpha -\text{amino acid}$ combines with the carboxylic acid group of $\alpha -\text{amino acid}$ to form a peptide bond by the removal of a water molecule. This is the case when the molecule is having only one peptide bond. This is shown below:
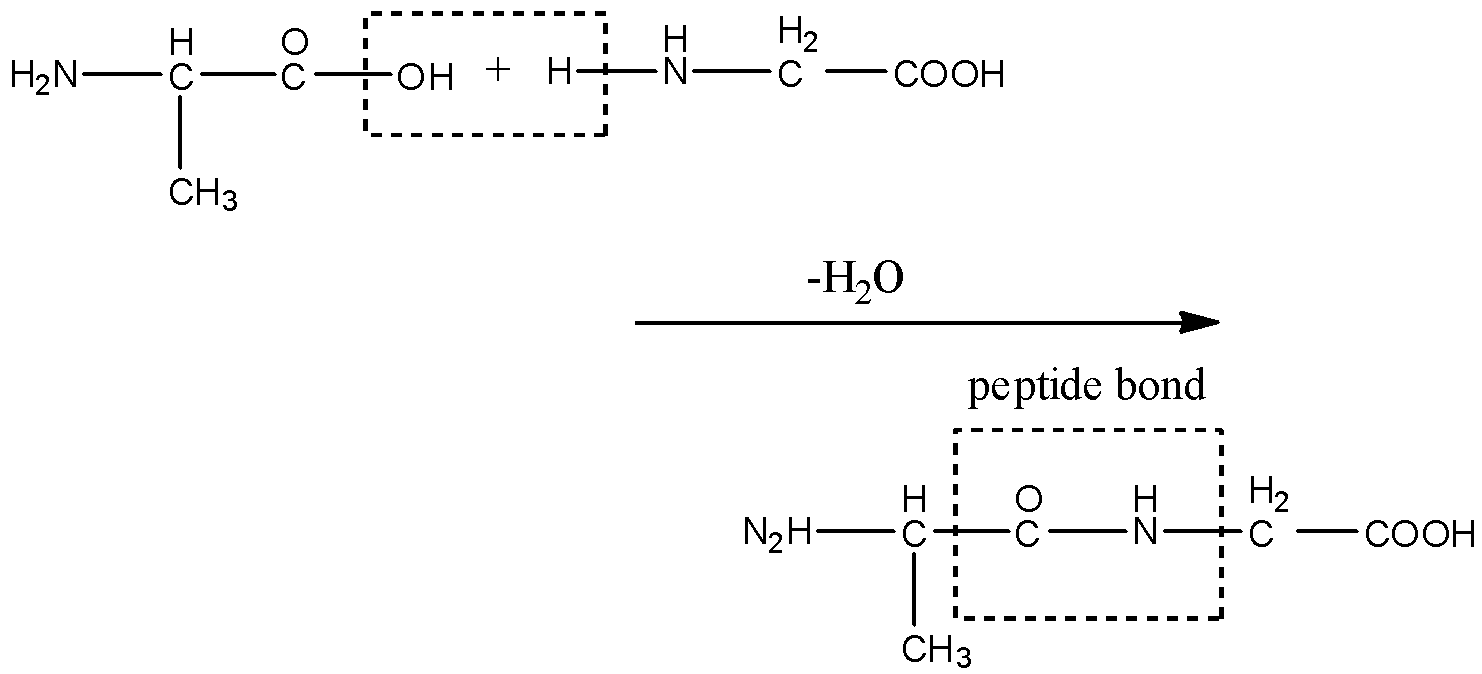
Tripeptide bond means the molecules have three peptide bonds. In which three $\alpha -\text{amino acid}$units are joined together. The amine group of one $\alpha -\text{amino acid}$is joined to the carboxylic acid group of the second $\alpha -\text{amino acid}$, the amine group of second $\alpha -\text{amino acid}$is joined to the carboxylic acid group of the third $\alpha -\text{amino acid}$.
So the formula of glycine is given below:
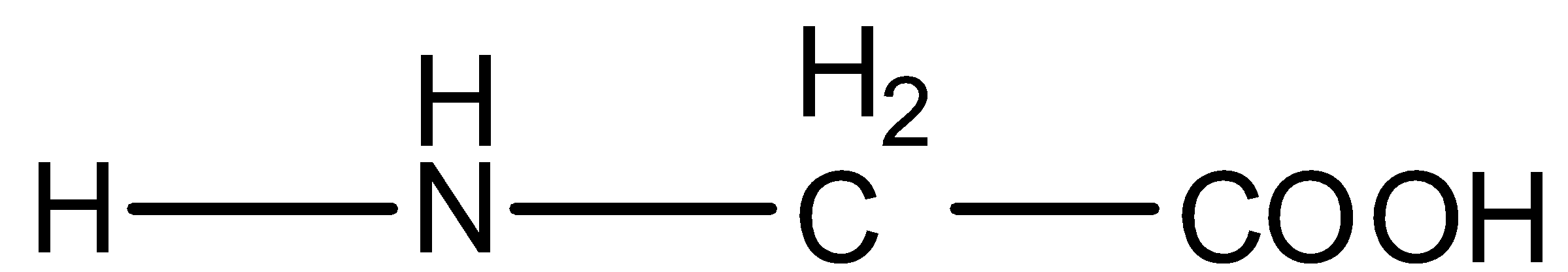
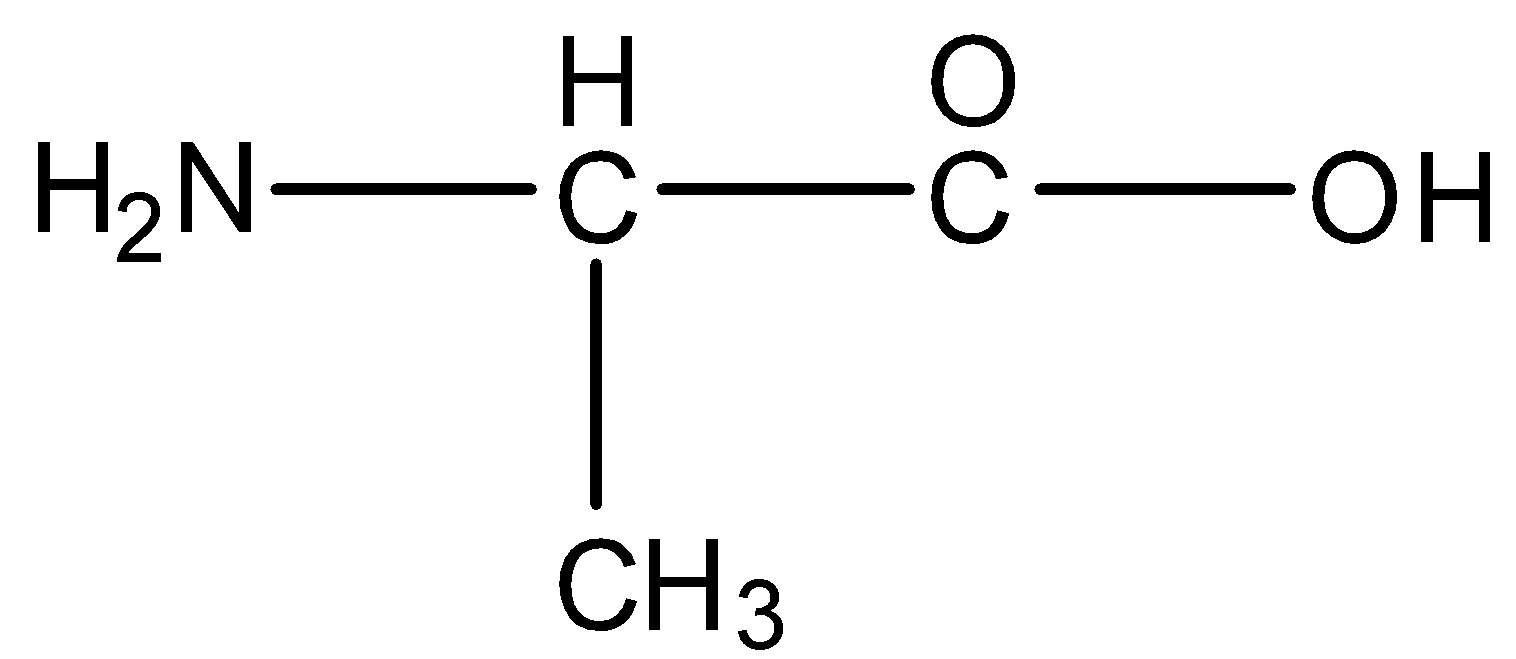
The formula of alanine is given below:
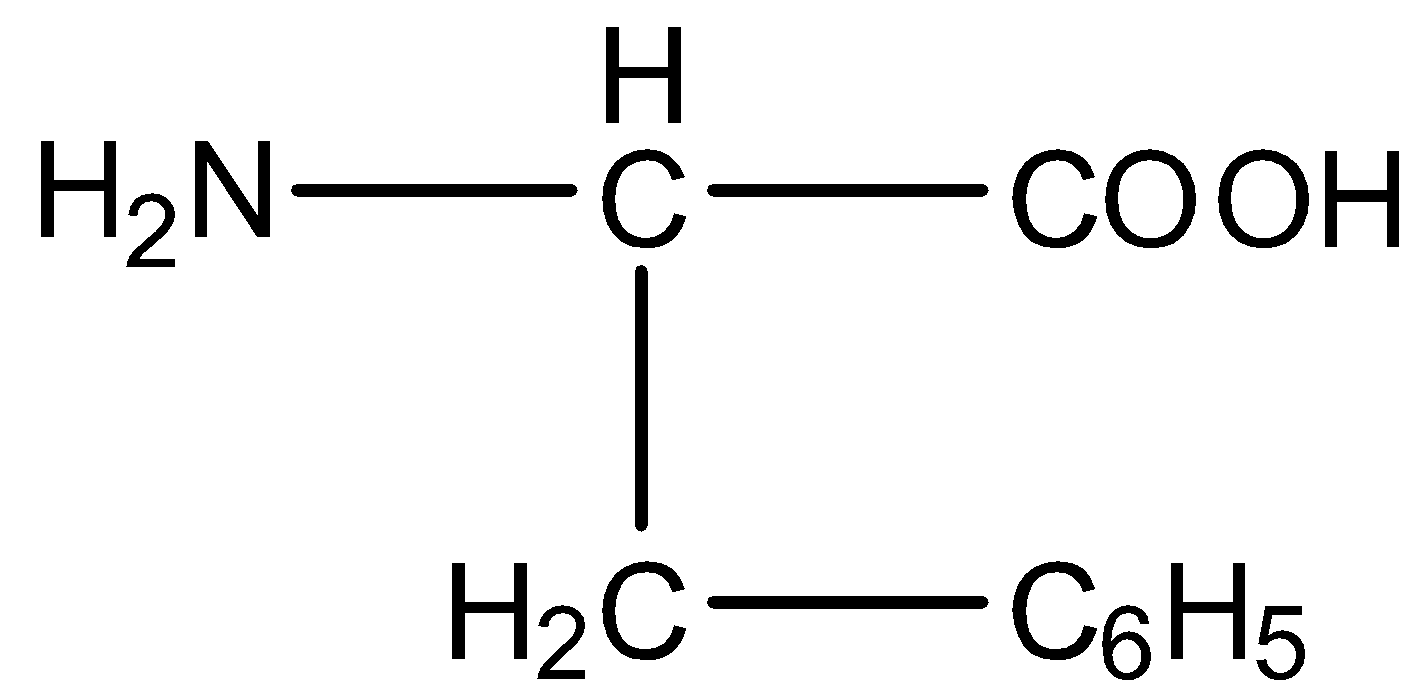
The formula of phenylalanine is given below:
So these three molecules can combine with themselves in six forms to make a tripeptide bond.
So, the correct answer is “Option C”.
Note: The peptide bond is also known as peptide linkage. If it is dipeptide then the molecule has two peptide bonds, if the molecule is tetrapeptide then the molecule has four peptide bonds. Polypeptide molecules have a large number of molecules and these are called proteins.
Complete step by step answer:
We know that an $\alpha -\text{amino acid}$ joins to another or the same $\alpha -\text{amino acid}$ by a bond called a peptide bond. $\alpha -\text{amino acid}$have two groups at its terminal carbon atoms i.e., an amine group and a carboxylic acid group. The amine group of one $\alpha -\text{amino acid}$ combines with the carboxylic acid group of $\alpha -\text{amino acid}$ to form a peptide bond by the removal of a water molecule. This is the case when the molecule is having only one peptide bond. This is shown below:

Tripeptide bond means the molecules have three peptide bonds. In which three $\alpha -\text{amino acid}$units are joined together. The amine group of one $\alpha -\text{amino acid}$is joined to the carboxylic acid group of the second $\alpha -\text{amino acid}$, the amine group of second $\alpha -\text{amino acid}$is joined to the carboxylic acid group of the third $\alpha -\text{amino acid}$.
So the formula of glycine is given below:
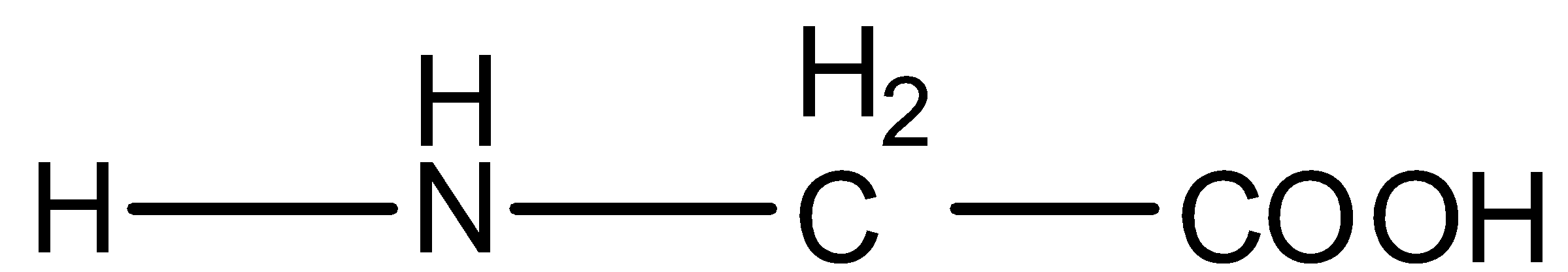
The formula of alanine is given below:

The formula of phenylalanine is given below:

So these three molecules can combine with themselves in six forms to make a tripeptide bond.
So, the correct answer is “Option C”.
Note: The peptide bond is also known as peptide linkage. If it is dipeptide then the molecule has two peptide bonds, if the molecule is tetrapeptide then the molecule has four peptide bonds. Polypeptide molecules have a large number of molecules and these are called proteins.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




