
Tropolone is an example of:
A. Benzenoid aromatic compound
B. non-benzenoid aromatic compound
C. alicyclic compound
D. heterocyclic aromatic compound
Answer
497.1k+ views
Hint: Tropolone is an organic compound. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor.
Complete answer:
For knowing each of the given compounds we must know the structure of tropolone. Therefore the structure of tropolone is,
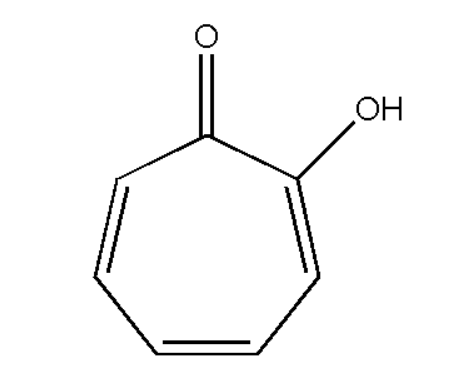
This is the basic structure of a tropolone. Its molecular formula is \[{{\text{C}}_7}{{\text{H}}_6}{{\text{O}}_2}\] having seven carbon atoms, six hydrogen atoms and two oxygen atoms. Now we will discuss each type of compound given and choose the right one. At first we had a benzenoid aromatic compound. These compounds are those which have benzene ring and they are aromatic in nature. Then we have, non-benzenoid aromatic compound. These are those compounds which do not have any benzene ring but they are aromatic in nature. Alicyclic compounds are those compounds which can be saturated or unsaturated but they do not show aromatic character. Thus these compounds are not aromatic in nature. Heterocyclic aromatic compounds are those which have more than one carbon atom but they are replaced by some group of atoms like nitrogen, oxygen and sulphur. Thus on observing each type of compound we can observe that tropolene is a non-benzenoid aromatic compound.
Hence the correct option is B.
Note:
Aromatic compounds are those which show aromaticity. We can also say that these compounds show resonance due to conjugate pi bond configuration which can be easily seen in tropolene. We can relate aromatic compounds to compounds which show resonance in its structure.
Complete answer:
For knowing each of the given compounds we must know the structure of tropolone. Therefore the structure of tropolone is,

This is the basic structure of a tropolone. Its molecular formula is \[{{\text{C}}_7}{{\text{H}}_6}{{\text{O}}_2}\] having seven carbon atoms, six hydrogen atoms and two oxygen atoms. Now we will discuss each type of compound given and choose the right one. At first we had a benzenoid aromatic compound. These compounds are those which have benzene ring and they are aromatic in nature. Then we have, non-benzenoid aromatic compound. These are those compounds which do not have any benzene ring but they are aromatic in nature. Alicyclic compounds are those compounds which can be saturated or unsaturated but they do not show aromatic character. Thus these compounds are not aromatic in nature. Heterocyclic aromatic compounds are those which have more than one carbon atom but they are replaced by some group of atoms like nitrogen, oxygen and sulphur. Thus on observing each type of compound we can observe that tropolene is a non-benzenoid aromatic compound.
Hence the correct option is B.
Note:
Aromatic compounds are those which show aromaticity. We can also say that these compounds show resonance due to conjugate pi bond configuration which can be easily seen in tropolene. We can relate aromatic compounds to compounds which show resonance in its structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




