
What is true for ${\left[ {Fe{{(CN)}_6}} \right]^{3 - }}$ and ${\left[ {Fe{F_6}} \right]^{3 - }}$?
A. Both are paramagnetic
B. Only ${\left[ {Fe{{(CN)}_6}} \right]^{3 - }}$ is paramagnetic
C. Only ${\left[ {Fe{F_6}} \right]^{3 - }}$ is paramagnetic
D. Both are diamagnetic
Answer
501.3k+ views
Hint: In coordination chemistry, the complex compounds in which all the electrons of the central metal atom are paired will tend to show diamagnetic behaviour while the compound in which the central metal atom consists of one or more than one unpaired electron is known as paramagnetic.
Complete answer:
An electrostatic model named crystal field theory allows us to understand certain aspects of a complex like magnetic behaviour, colour properties, electronic spectra, etc. CFT focuses on the nonbonding electrons on the central metal ion in the complex instead of metal ligand bonds. This theory was given by H. Bethe and V. Vleck and main points of this theory are as follows:
1. The bonding between a central metal atom and the ligand in a complex is considered as purely ionic.
2. The central metal atom is regarded as a positive charge while the ligands are regarded as a negative charge and are attracted towards the central metal atom.
3. The electrons of the central metal atom and those of ligands repel each other and these repulsive forces are responsible for the splitting of d-orbitals of the central metal atom.
4. The effect of ligands is particularly marked on the d-electrons and it depends on their number, arrangement and nature which determine the crystal field of the complex.
5. The colour of the metal complex and its magnetic properties can be explained in terms of the transition of electrons and splitting of d-orbitals in different crystal fields.
Now, for the given complexes, the central metal ion is same i.e., $F{e^{3 + }}$ but differ in ligands and we know that a pattern of increasing sigma donors is as follows:
$X < O < N < C$
Where X represents halogens. Thus, we can say that $C{N^ - }$ is the strong field ligand due to high tendency of sigma donation and will form a low spin complex whereas ${F^ - }$ is the weak field ligand and will form a high spin complex. Therefore, the splitting of d-orbital in $F{e^{3 + }}$ ion in both the metal complexes is represented as follows:
Splitting of d orbital of $F{e^{3 + }}$ in ${\left[ {Fe{{(CN)}_6}} \right]^{3 - }}$:
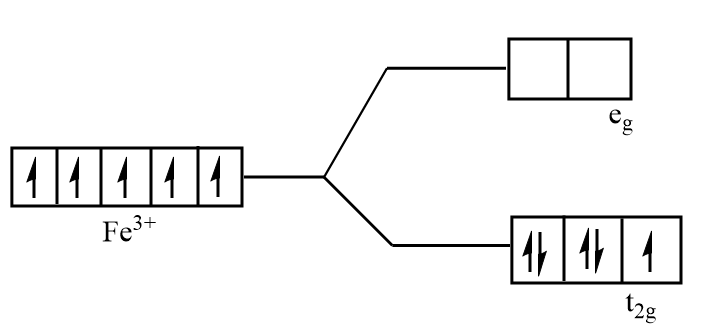
As $C{N^ - }$ is a strong field ligand, so the pairing energy is less than the energy separation between the two levels. Thus, pairing of electrons takes place but as there is one electron left unpaired in the ${t_{2g}}$ orbital so the complex is paramagnetic.
Splitting of d orbital of $F{e^{3 + }}$ in ${\left[ {Fe{F_6}} \right]^{3 - }}$:
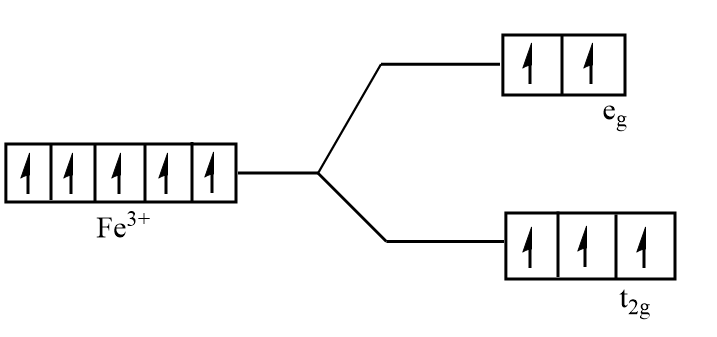
As ${F^ - }$ is a weak field ligand, so the pairing energy is more than the energy separation between the two levels. Thus, pairing of electrons will not take place and all the electrons will be unpaired. So, the complex is paramagnetic.
Hence, Both the given complexes are paramagnetic. So, option (A) is the correct answer.
Note:
It is important to note that in diamagnetic complexes, there are no unpaired electrons to show transitions between different energy levels while in paramagnetic complexes, the unpaired electrons can easily undergo transitions. Thus, diamagnetic complexes are not coloured whereas the paramagnetic complexes show colours. Also, remember that the colour of a complex can also be explained on the basis of metal-ligand charge transfer.
Complete answer:
An electrostatic model named crystal field theory allows us to understand certain aspects of a complex like magnetic behaviour, colour properties, electronic spectra, etc. CFT focuses on the nonbonding electrons on the central metal ion in the complex instead of metal ligand bonds. This theory was given by H. Bethe and V. Vleck and main points of this theory are as follows:
1. The bonding between a central metal atom and the ligand in a complex is considered as purely ionic.
2. The central metal atom is regarded as a positive charge while the ligands are regarded as a negative charge and are attracted towards the central metal atom.
3. The electrons of the central metal atom and those of ligands repel each other and these repulsive forces are responsible for the splitting of d-orbitals of the central metal atom.
4. The effect of ligands is particularly marked on the d-electrons and it depends on their number, arrangement and nature which determine the crystal field of the complex.
5. The colour of the metal complex and its magnetic properties can be explained in terms of the transition of electrons and splitting of d-orbitals in different crystal fields.
Now, for the given complexes, the central metal ion is same i.e., $F{e^{3 + }}$ but differ in ligands and we know that a pattern of increasing sigma donors is as follows:
$X < O < N < C$
Where X represents halogens. Thus, we can say that $C{N^ - }$ is the strong field ligand due to high tendency of sigma donation and will form a low spin complex whereas ${F^ - }$ is the weak field ligand and will form a high spin complex. Therefore, the splitting of d-orbital in $F{e^{3 + }}$ ion in both the metal complexes is represented as follows:
Splitting of d orbital of $F{e^{3 + }}$ in ${\left[ {Fe{{(CN)}_6}} \right]^{3 - }}$:

As $C{N^ - }$ is a strong field ligand, so the pairing energy is less than the energy separation between the two levels. Thus, pairing of electrons takes place but as there is one electron left unpaired in the ${t_{2g}}$ orbital so the complex is paramagnetic.
Splitting of d orbital of $F{e^{3 + }}$ in ${\left[ {Fe{F_6}} \right]^{3 - }}$:

As ${F^ - }$ is a weak field ligand, so the pairing energy is more than the energy separation between the two levels. Thus, pairing of electrons will not take place and all the electrons will be unpaired. So, the complex is paramagnetic.
Hence, Both the given complexes are paramagnetic. So, option (A) is the correct answer.
Note:
It is important to note that in diamagnetic complexes, there are no unpaired electrons to show transitions between different energy levels while in paramagnetic complexes, the unpaired electrons can easily undergo transitions. Thus, diamagnetic complexes are not coloured whereas the paramagnetic complexes show colours. Also, remember that the colour of a complex can also be explained on the basis of metal-ligand charge transfer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




