
Two esters with the formula \[{\text{ }}{{\text{C}}_3}{{\text{H}}_6}{{\text{O}}_2}{\text{ }}\]
Answer
548.1k+ views
Hint:
Esters are the compound having the function group \[ - {\text{COOR}}\] . Here R is an alkyl group. One ethyl ester and one methyl ester will form.
Complete step by step solution:
Esters are the carboxylic acid derivative. The hydrogen attached with carboxylic acid is replaced by alkyl groups. One methyl group and one ethyl group can be replaced with hydrogen and hence two esters will form. The formula and name of both the esters are as follow:
The first ester formed is methyl acetate.
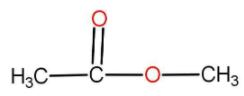
The formula has 3 carbon, 2 oxygen and 6 hydrogen atoms. Hence it fits the formula given.
The name of the ester is ethyl formate.
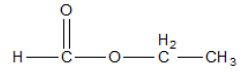
The formula has 3 carbons, 2 oxygen and 6 hydrogen atoms. Hence it also fits the formula given.
Additional information:
Esters are generally flammable liquids. Methyl acetate is also known as acetic acid, methyl ester or methyl ethanoate. It has a characteristic smell just like some true or nail paint remover smell it is used as a solvent. On reaction with sodium hydroxide strong acids it hydrolysed to form ethanol and acetic acid. When ethanol is reacted with formic acid ethyl formate is formed. It has a rum-like smell and is also present in raspberries. In nature ethyl acetate can be found in ant bodies and bee sting.
Note:
Esters can be prepared from esterification of carboxylic acid and alcohol or of carboxylic acid with epoxide. It is also from alkylation of carboxylate salts. Addition of carboxylic acid to alkene alkyne also yields esters. Esters with low molecular weight are used as fragrance and are found in essential oil and perfumes. They also have a sweet smell.
Esters are the compound having the function group \[ - {\text{COOR}}\] . Here R is an alkyl group. One ethyl ester and one methyl ester will form.
Complete step by step solution:
Esters are the carboxylic acid derivative. The hydrogen attached with carboxylic acid is replaced by alkyl groups. One methyl group and one ethyl group can be replaced with hydrogen and hence two esters will form. The formula and name of both the esters are as follow:
The first ester formed is methyl acetate.

The formula has 3 carbon, 2 oxygen and 6 hydrogen atoms. Hence it fits the formula given.
The name of the ester is ethyl formate.
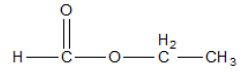
The formula has 3 carbons, 2 oxygen and 6 hydrogen atoms. Hence it also fits the formula given.
Additional information:
Esters are generally flammable liquids. Methyl acetate is also known as acetic acid, methyl ester or methyl ethanoate. It has a characteristic smell just like some true or nail paint remover smell it is used as a solvent. On reaction with sodium hydroxide strong acids it hydrolysed to form ethanol and acetic acid. When ethanol is reacted with formic acid ethyl formate is formed. It has a rum-like smell and is also present in raspberries. In nature ethyl acetate can be found in ant bodies and bee sting.
Note:
Esters can be prepared from esterification of carboxylic acid and alcohol or of carboxylic acid with epoxide. It is also from alkylation of carboxylate salts. Addition of carboxylic acid to alkene alkyne also yields esters. Esters with low molecular weight are used as fragrance and are found in essential oil and perfumes. They also have a sweet smell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




