
What type of glycosidic linkage is the following?
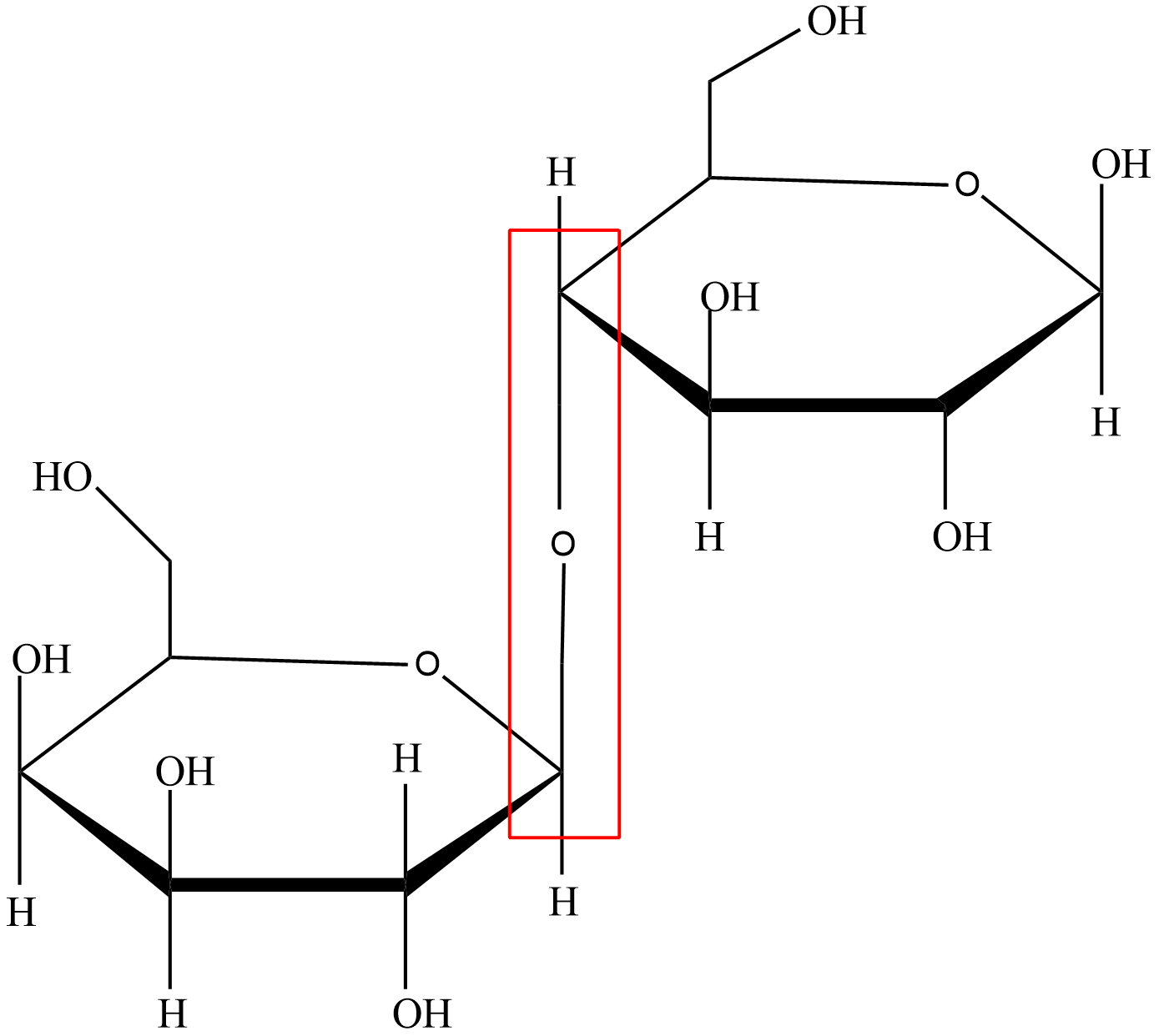
A.\[\alpha (1 \to 4')\]
B.\[\beta (1 \to 4')\]
C.\[\alpha (1 \to 6')\]
D.\[\beta (1 \to 6')\]

Answer
501.9k+ views
Hint: A glycosidic bond or glycosidic linkage is a type of covalent bond that bonds a carbohydrate molecule to another molecule which may or may not be a carbohydrate. It is an ether linkage (\[C - O - C\]) in which at least one carbon is part of a carbohydrate molecule. The compound having a glycosidic linkage or glycosidic bond is known as glycoside.
Complete answer:
This glycosidic linkage is formed between the hemiacetal and hemiketal group of a saccharide (or a compound which is derived from saccharide) and hydroxyl group like alcohol group of another compound.
We can differentiate alpha glycosidic linkage and beta glycosidic by the relative stereochemistry of the anomeric position and the stereocenter that is farthest from the first carbon. An alpha glycosidic linkage is formed between the carbons with the same stereochemistry. A beta glycosidic linkage is formed between the carbons having different stereochemistry.
Let’s look at the image that is given to us, we can observe that the both carbons of the two monosaccharide that are forming the linkage have different stereochemistry so this is a beta glycosidic linkage and the first carbon of one monosaccharide is bonded to fourth carbon of other monosaccharide so it is a \[1 \to 4'\]linkage. So, it is a \[\beta (1 \to 4')\] glycosidic linkage.
Hence, option (B) is the right answer.
Note:
Carefully observe the structure of monosaccharides to identify its type whether it is alpha or beta. Also mind the position of the carbons that are forming the glycosidic linkage as position is necessary to find the linkage type in that particular molecule.
Complete answer:
This glycosidic linkage is formed between the hemiacetal and hemiketal group of a saccharide (or a compound which is derived from saccharide) and hydroxyl group like alcohol group of another compound.
We can differentiate alpha glycosidic linkage and beta glycosidic by the relative stereochemistry of the anomeric position and the stereocenter that is farthest from the first carbon. An alpha glycosidic linkage is formed between the carbons with the same stereochemistry. A beta glycosidic linkage is formed between the carbons having different stereochemistry.
Let’s look at the image that is given to us, we can observe that the both carbons of the two monosaccharide that are forming the linkage have different stereochemistry so this is a beta glycosidic linkage and the first carbon of one monosaccharide is bonded to fourth carbon of other monosaccharide so it is a \[1 \to 4'\]linkage. So, it is a \[\beta (1 \to 4')\] glycosidic linkage.
Hence, option (B) is the right answer.
Note:
Carefully observe the structure of monosaccharides to identify its type whether it is alpha or beta. Also mind the position of the carbons that are forming the glycosidic linkage as position is necessary to find the linkage type in that particular molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




