
Using formal charge, which of the following would be the least likely structure for dinitrogen monoxide based on the knowledge that oxygen is not the central atom?
A.\[N - N = O\]
B.\[N = N = O\]
C.
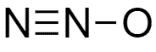
D.All the structures are equally likely as drawn
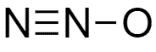
Answer
519.3k+ views
Hint: Basically, formal charge is the theoretical charge which is given to the atoms of the molecule on the basis of the assumption that electrons are shared equally by the atoms in the chemical bond without considering their electronegativity. In the structure, formal charge tells us about the quality of dot structure.
Complete answer:
Now, we know that for a single structure multiple dot structures can be easily drawn, so, formal charge is the best method in order to distinguish between the structures which one is the best possible dot structure.
If you can easily draw several dot structures for a particular molecule, then the question arises that how can we distinguish which one is the steadiest dot structure? Formal charge. Formal charge is the best method to distinguish. For that there are few rules such as:
Structures having the minimum size of the formal charge are considered to be stable.
If the electronegative atom is present, then they will have negative formal charge over it.
Neighboring atoms have inverse formal charge.
Now, how to calculate formal charge of a molecule?
Formal charge for every atom in the molecule can be easily calculated using a formula, i.e.
Formal Charge = valence electrons $ - $ $0.5 \times $Bonding electrons $ - $ Nonbonding electrons
Now, from the given options that structure will be steadiest on which there will be minimum size of the formal charge so,
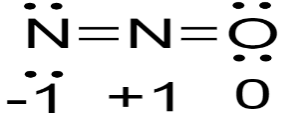
So, as we can see the formal charge is minimal here in this, so this is the steadiest structure as compared to the other structures.
So, the correct option is B) \[N = N = O\]
Note:
For an atom, formal charge is determined on the basis of the amount of the valence electrons and the amount of electron which is surrounding the atom and forming a bond with the molecule.
Complete answer:
Now, we know that for a single structure multiple dot structures can be easily drawn, so, formal charge is the best method in order to distinguish between the structures which one is the best possible dot structure.
If you can easily draw several dot structures for a particular molecule, then the question arises that how can we distinguish which one is the steadiest dot structure? Formal charge. Formal charge is the best method to distinguish. For that there are few rules such as:
Structures having the minimum size of the formal charge are considered to be stable.
If the electronegative atom is present, then they will have negative formal charge over it.
Neighboring atoms have inverse formal charge.
Now, how to calculate formal charge of a molecule?
Formal charge for every atom in the molecule can be easily calculated using a formula, i.e.
Formal Charge = valence electrons $ - $ $0.5 \times $Bonding electrons $ - $ Nonbonding electrons
Now, from the given options that structure will be steadiest on which there will be minimum size of the formal charge so,
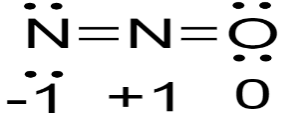
So, as we can see the formal charge is minimal here in this, so this is the steadiest structure as compared to the other structures.
So, the correct option is B) \[N = N = O\]
Note:
For an atom, formal charge is determined on the basis of the amount of the valence electrons and the amount of electron which is surrounding the atom and forming a bond with the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




