
Vulcanization makes rubber:
The question has multiple correct options
A) More elastic
B) Soluble in organic solvent
C) Crystalline
D) More stiff
Answer
567k+ views
Hint: The natural rubber is so sticky at high temperature and brittle at low temperature they can be used only in a very narrow range of temperature.
- In the vulcanization process of natural rubber we generally use Sulphur as the chemical to alter the properties of rubber.
Complete Solution :
- In the branch of organic chemistry, we study a range of products called polymers and they find applications in almost every industry and they are growing as a very important part in our day to day life.
They have high industrial value.
- The polymers are compounds with higher molecular masses and formed by the repeated addition of a subunit generally called as the monomers. The monomers adding on repeatedly and undergoing processes like addition, condensation reactions etc. form the polymers.
So here in the question we are asked about a process called vulcanization. The term vulcanization is generally related to polymer rubber. The natural rubber which is obtained from the rubber tree in the form of latex is unfit for the industrial purpose.
- They are too sticky at higher temperatures and brittle at lower temperatures which limits their usage.
- They are not resistant towards the attack of chemicals, they also show high water absorbing capacity.
- These properties limit its industrial use and to enhance the new properties that extend its industrial value we use a process called vulcanization and it is one of the most important processes in the production of rubber for commercial purposes.
- In this method we add a chemical substance, say the vulcanizing agent generally used is Sulphur. S along with the polymer and is heated at a temperature range of 375 K to 415 K, which improves the quality of natural rubber.
- And during this process of vulcanization the sulphur atom reacts with the polymer and forms the three dimensional cross linkage with the polymer (Natural rubber).
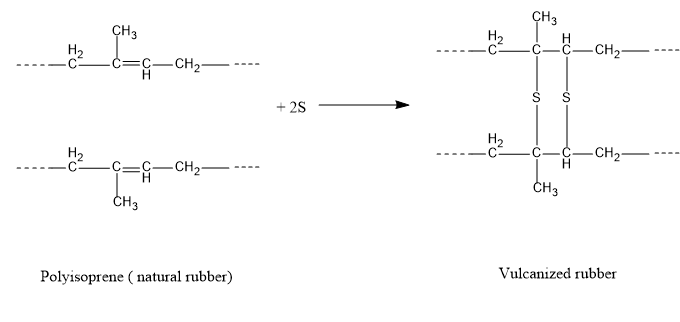
The natural rubber is called polyisoprene, since the monomer of rubber is isoprene.
By doing this vulcanization process the quality of the rubber is improved and they can be used in a wide range of temperature, they are hard and tough. Due to the cross-linkage, the elasticity of the rubber has increased and its mechanical properties have tuned a way better. They possess high tensile strength and they are highly resistive towards the attack of chemicals.
So, the correct answer is “Option A and D”.
Note: The other vulcanizing agents that are used in this process are Selenium, oxygen, tellurium etc.
- The monomer of rubber isoprene has its IUPAC names as 2-methyl-1, 3-butadiene.
- There are two types of rubber –natural rubber and synthetic rubber.
- Synthetic rubbers are artificially man-made rubbers.
- In the vulcanization process of natural rubber we generally use Sulphur as the chemical to alter the properties of rubber.
Complete Solution :
- In the branch of organic chemistry, we study a range of products called polymers and they find applications in almost every industry and they are growing as a very important part in our day to day life.
They have high industrial value.
- The polymers are compounds with higher molecular masses and formed by the repeated addition of a subunit generally called as the monomers. The monomers adding on repeatedly and undergoing processes like addition, condensation reactions etc. form the polymers.
So here in the question we are asked about a process called vulcanization. The term vulcanization is generally related to polymer rubber. The natural rubber which is obtained from the rubber tree in the form of latex is unfit for the industrial purpose.
- They are too sticky at higher temperatures and brittle at lower temperatures which limits their usage.
- They are not resistant towards the attack of chemicals, they also show high water absorbing capacity.
- These properties limit its industrial use and to enhance the new properties that extend its industrial value we use a process called vulcanization and it is one of the most important processes in the production of rubber for commercial purposes.
- In this method we add a chemical substance, say the vulcanizing agent generally used is Sulphur. S along with the polymer and is heated at a temperature range of 375 K to 415 K, which improves the quality of natural rubber.
- And during this process of vulcanization the sulphur atom reacts with the polymer and forms the three dimensional cross linkage with the polymer (Natural rubber).

The natural rubber is called polyisoprene, since the monomer of rubber is isoprene.
By doing this vulcanization process the quality of the rubber is improved and they can be used in a wide range of temperature, they are hard and tough. Due to the cross-linkage, the elasticity of the rubber has increased and its mechanical properties have tuned a way better. They possess high tensile strength and they are highly resistive towards the attack of chemicals.
So, the correct answer is “Option A and D”.
Note: The other vulcanizing agents that are used in this process are Selenium, oxygen, tellurium etc.
- The monomer of rubber isoprene has its IUPAC names as 2-methyl-1, 3-butadiene.
- There are two types of rubber –natural rubber and synthetic rubber.
- Synthetic rubbers are artificially man-made rubbers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




