
What are directing groups?
Answer
478.5k+ views
Hint: The nucleophilicity of the ring will be affected in the opposite way by an electron-withdrawing group (EWG). The EWG deactivates groups by decreasing electron density from a system, making it less reactive in this kind of reaction. EDGs and EWGs also define the locations on the aromatic ring where substitution reactions are most likely to occur (relative to themselves); this characteristic is essential in organic synthesis processes.
Complete answer:
Existing substituent groups on the aromatic ring impact the overall reaction rate or have a directing effect on the positional isomer of the products produced in an electrophilic aromatic substitution process. An atom or functional group that contributes some of its electron density into a conjugated system via resonance or inductive effects (called +M or +I effects, respectively) to make the system more nucleophilic is known as an electron-donating or electron-releasing group. An aromatic ring with such a group attached is more likely to engage in electrophilic substitution reactions as a result of these electrical effects. As a result, EDGs are frequently referred to as activating groups.
A directing group (DG) is a substituent on a molecule or ion that promotes reactions by interacting with a reagent in organic chemistry. A "coordinating moiety (an "internal ligand"), which guides a metal catalyst into the vicinity of a particular C–H bond," is how the word is generally applied to C-H activation of hydrocarbons. The ketone group in acetophenone, for example, is the DG in the Murai reaction.
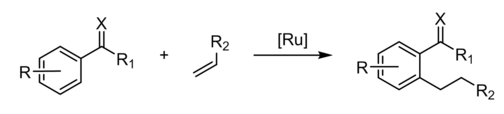
The Murai reaction is linked to directed ortho metalation, which is commonly used to lithiated substituted aromatic rings.

Note:
Because directing groups are ligands, their efficiency is proportional to their metal affinity. Common functional groups, like ketones, are often weak ligands and hence poor DGs. A temporary directing group is used to overcome this problem. Transient DGs use a Schiff base condensation to reversibly transform weak DGs (e.g., ketones) into strong DGs (e.g., imines). The imine can hydrolyze after performing their function as DGs, regenerating the ketone and amine.
Complete answer:
Existing substituent groups on the aromatic ring impact the overall reaction rate or have a directing effect on the positional isomer of the products produced in an electrophilic aromatic substitution process. An atom or functional group that contributes some of its electron density into a conjugated system via resonance or inductive effects (called +M or +I effects, respectively) to make the system more nucleophilic is known as an electron-donating or electron-releasing group. An aromatic ring with such a group attached is more likely to engage in electrophilic substitution reactions as a result of these electrical effects. As a result, EDGs are frequently referred to as activating groups.
A directing group (DG) is a substituent on a molecule or ion that promotes reactions by interacting with a reagent in organic chemistry. A "coordinating moiety (an "internal ligand"), which guides a metal catalyst into the vicinity of a particular C–H bond," is how the word is generally applied to C-H activation of hydrocarbons. The ketone group in acetophenone, for example, is the DG in the Murai reaction.

The Murai reaction is linked to directed ortho metalation, which is commonly used to lithiated substituted aromatic rings.

Note:
Because directing groups are ligands, their efficiency is proportional to their metal affinity. Common functional groups, like ketones, are often weak ligands and hence poor DGs. A temporary directing group is used to overcome this problem. Transient DGs use a Schiff base condensation to reversibly transform weak DGs (e.g., ketones) into strong DGs (e.g., imines). The imine can hydrolyze after performing their function as DGs, regenerating the ketone and amine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




