
What family is helium in?
Answer
528.6k+ views
Hint: The periodic table has all the elements arranged in accordance with their atomic numbers. The atomic number of elements leads to the electronic configuration of elements. Through electronic configuration, the elements can be classified according to the number of electrons in the orbitals.
Complete answer:
The modern periodic table consists of elements arranged in accordance with the modern periodic law that states, ‘the physical and chemical properties of elements are the periodic function of their atomic numbers’. This law clearly states that elements in the periodic table are arranged in order of their increasing atomic number.
In the periodic table, each element consists of an electron configuration that decides the nature and properties of that element. The atomic number is the number of electrons, and the electronic configuration is the filling of electrons into different orbitals, like, s, p, d, and f.
From the periodic table, we can tell the position of helium,
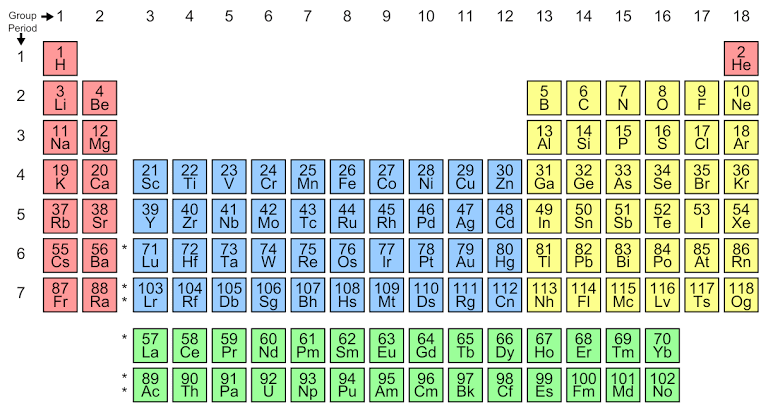
Helium, occupies, second number with 2 as the atomic number, so according to the Aufbau principle, the electron configuration of helium will be $He=1{{s}^{2}}$ , this shows it has a fully filled electron configuration, that makes it a member of the noble gases of group 18. The noble gases consist of all the orbitals as fully filled, and thus they are inert gases.
Hence, helium comes under the family of noble gases.
Note:
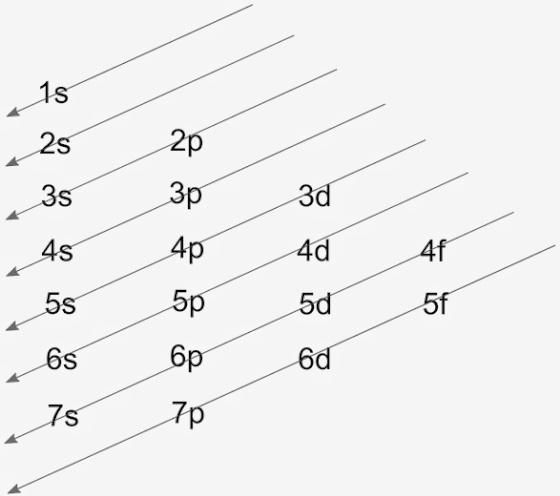
There are 5 families in the periodic table, which are, alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. The filling of electrons takes place from the lower energy level to the higher energy levels according to the Aufbau principle, which is the name of the scientist. The filling is according to Aufbau diagram as:
Complete answer:
The modern periodic table consists of elements arranged in accordance with the modern periodic law that states, ‘the physical and chemical properties of elements are the periodic function of their atomic numbers’. This law clearly states that elements in the periodic table are arranged in order of their increasing atomic number.
In the periodic table, each element consists of an electron configuration that decides the nature and properties of that element. The atomic number is the number of electrons, and the electronic configuration is the filling of electrons into different orbitals, like, s, p, d, and f.
From the periodic table, we can tell the position of helium,

Helium, occupies, second number with 2 as the atomic number, so according to the Aufbau principle, the electron configuration of helium will be $He=1{{s}^{2}}$ , this shows it has a fully filled electron configuration, that makes it a member of the noble gases of group 18. The noble gases consist of all the orbitals as fully filled, and thus they are inert gases.
Hence, helium comes under the family of noble gases.
Note:
There are 5 families in the periodic table, which are, alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. The filling of electrons takes place from the lower energy level to the higher energy levels according to the Aufbau principle, which is the name of the scientist. The filling is according to Aufbau diagram as:

Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




