
What is a Biuret test?
Answer
599.7k+ views
Hint: Before attempting this question, one must have prior knowledge of oxidation and reduction, and how it leads to change in color, especially in the case of $C{u^{2 \oplus }}$ and ion.
Complete answer:
> The Piotrowski’s test, or as it is more commonly known, Biuret test. This test is used, when we have to detect the presence of peptide bonds in an alkaline solution.
> Interestingly, the biuret test does not use the reagent biuret. Instead, it uses a mixture of sodium hydroxide and hydrated Copper (II) Sulfate$C{u^ \oplus }$ as reagents. Potassium Sodium Tartrate is also used to stabilize the chelate formed.\[C{u^{2 \oplus }}\]
> Reaction: In the presence of\[NaOH\], \[C{u^{2 \oplus }}\] from hydrated Copper (II) Sulfate$(CuS{O_4}.x{H_2}O)$ is released. Then combine with peptide molecules to form a chelate as shown below.
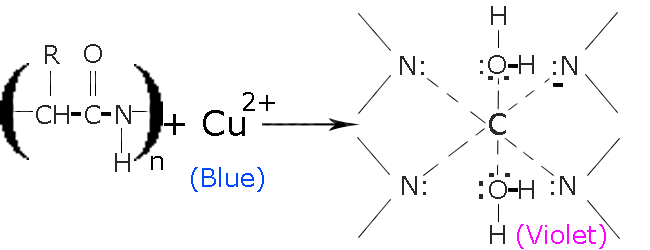
> The reaction occurs in two steps:-
1) Copper(II) binds with nitrogen, that are present in the protein’s peptide chains
2) In the 2nd step Copper (II) is reduced to Copper (I), which gives the solution its iconic light-purple color.
> The biuret solution is blue in color and turns light-purple, when it reacts with compounds containing peptide bonds.
> The biuret can also be used to measure the concentration of proteins, by using the Beer-Lambert law i.e. the intensity of the color (absorption of light at 540nm) is directly proportional to the protein concentration
Note: Buffers, like Ammonia interfere with the reaction, therefore this test is not suitable for protein samples purified from ammonia sulfate precipitation. The $NaOH$solution used should be concentrated and then followed by a few drops of hydrated Copper (II) Sulfate$(CuS{O_4}.x{H_2}O)$.
Complete answer:
> The Piotrowski’s test, or as it is more commonly known, Biuret test. This test is used, when we have to detect the presence of peptide bonds in an alkaline solution.
> Interestingly, the biuret test does not use the reagent biuret. Instead, it uses a mixture of sodium hydroxide and hydrated Copper (II) Sulfate$C{u^ \oplus }$ as reagents. Potassium Sodium Tartrate is also used to stabilize the chelate formed.\[C{u^{2 \oplus }}\]
> Reaction: In the presence of\[NaOH\], \[C{u^{2 \oplus }}\] from hydrated Copper (II) Sulfate$(CuS{O_4}.x{H_2}O)$ is released. Then combine with peptide molecules to form a chelate as shown below.

> The reaction occurs in two steps:-
1) Copper(II) binds with nitrogen, that are present in the protein’s peptide chains
2) In the 2nd step Copper (II) is reduced to Copper (I), which gives the solution its iconic light-purple color.
> The biuret solution is blue in color and turns light-purple, when it reacts with compounds containing peptide bonds.
> The biuret can also be used to measure the concentration of proteins, by using the Beer-Lambert law i.e. the intensity of the color (absorption of light at 540nm) is directly proportional to the protein concentration
Note: Buffers, like Ammonia interfere with the reaction, therefore this test is not suitable for protein samples purified from ammonia sulfate precipitation. The $NaOH$solution used should be concentrated and then followed by a few drops of hydrated Copper (II) Sulfate$(CuS{O_4}.x{H_2}O)$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




