
What is a coordination polyhedron?
Answer
507k+ views
Hint : Coordinate compounds are the molecules that possess a central metal atom surrounded by ligands through coordinate or dative bond. Here central metal acts as lewis acid and accepts the electron from ligands that act like lewis base. Alfred Werner was the first person to propose his insight on the coordination compounds. These compounds retain their identity in crystal lattice as well as solution or molten state. Example- $ {[Ni{(N{H_3})_6}]^{2 + }} $ is a popular example in which nickel acts as a central metal atom while Ammonia acts as ligand.
Complete Step By Step Answer:
Werner explained that coordination compounds possess 2 types of valency-Primary and secondary valency. Primary valency is ionisable, shows the oxidation state or charge on the complex and shown by dashed line. Secondary valency is non-ionisable, it decides geometry or shape of molecule and shown by solid line. Primary valency is non-directional while secondary valency is directional.
Example- $ [Ni{(N{H_3})_6}]C{l_2} $ in this case Ammonia is shown by solid lines (secondary valency) while chlorine is shown by dotted lines (primary valency).The species within the square bracket is called coordination entities or complex and outside the square bracket ions are called counter ions.
Central metal atom is generally 1st, 2nd or 3rd period transition elements. The ligands can be all those molecules which have either lone pair or negative charge to donate.
Monodentate ligand-Only 1 donor site in the ligand. For Example- Ammonia $ (N{H_3}) $ ,Carbonyl $ (CO) $ ,Phosphine $ (P{H_3}) $ etc.
Bidentate ligand-2 donor site in the ligand. For Example-oxalate $ ({C_2}{O_4}^{2 - }) $
Polydentate ligand-More than 2 donor atoms. For Example-EDTA (Ethylene diamine tetra acetate) is hexadentate ligand.
Here Coordination polyhedron refers to the spatial geometry or arrangements of ligands around the central metal atom of the coordination compounds i.e. these ligands are attached to the central atom in space in a definite direction. It may be either octahedral, tetrahedral, trigonal bipyramidal, square pyramidal or of square planar shapes.
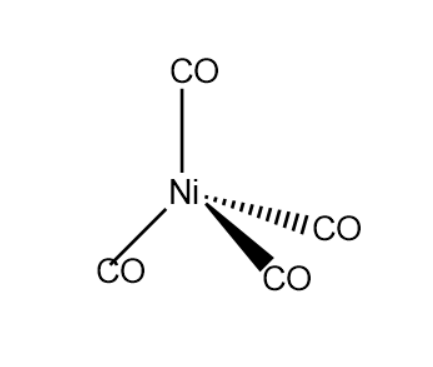
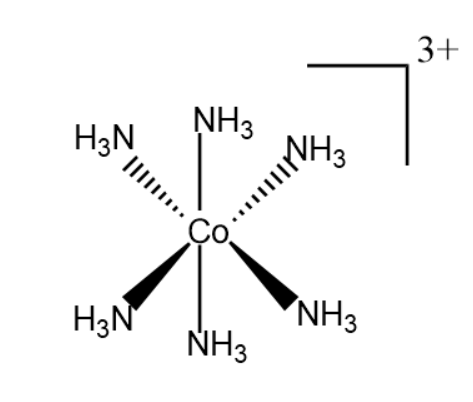
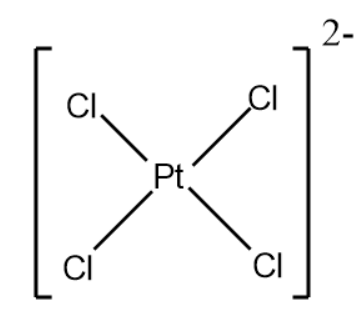
Example- $ Ni{(CO)_4} $ is of tetrahedral geometry, $ {[Co{(N{H_3})_6}]^{3 + }} $ is of octahedral geometry, $ {[PtC{l_4}]^{2 - }} $ is of square planar geometry etc.
Note :
Ligands mostly act as donors while sometimes ligand can accept the electrons from metal by pi back bonding in $ {\pi ^*} $ Molecular orbital or vacant d orbital known as pi acid ligand and pi base Metal. Complexes can either be homoleptic or heteroleptic.Homoleptic means Metal surrounded by the same type of ligands and heteroleptic means metal surrounded by 2 or more types of ligands.
Complete Step By Step Answer:
Werner explained that coordination compounds possess 2 types of valency-Primary and secondary valency. Primary valency is ionisable, shows the oxidation state or charge on the complex and shown by dashed line. Secondary valency is non-ionisable, it decides geometry or shape of molecule and shown by solid line. Primary valency is non-directional while secondary valency is directional.
Example- $ [Ni{(N{H_3})_6}]C{l_2} $ in this case Ammonia is shown by solid lines (secondary valency) while chlorine is shown by dotted lines (primary valency).The species within the square bracket is called coordination entities or complex and outside the square bracket ions are called counter ions.
Central metal atom is generally 1st, 2nd or 3rd period transition elements. The ligands can be all those molecules which have either lone pair or negative charge to donate.
Monodentate ligand-Only 1 donor site in the ligand. For Example- Ammonia $ (N{H_3}) $ ,Carbonyl $ (CO) $ ,Phosphine $ (P{H_3}) $ etc.
Bidentate ligand-2 donor site in the ligand. For Example-oxalate $ ({C_2}{O_4}^{2 - }) $
Polydentate ligand-More than 2 donor atoms. For Example-EDTA (Ethylene diamine tetra acetate) is hexadentate ligand.
Here Coordination polyhedron refers to the spatial geometry or arrangements of ligands around the central metal atom of the coordination compounds i.e. these ligands are attached to the central atom in space in a definite direction. It may be either octahedral, tetrahedral, trigonal bipyramidal, square pyramidal or of square planar shapes.
Example- $ Ni{(CO)_4} $ is of tetrahedral geometry, $ {[Co{(N{H_3})_6}]^{3 + }} $ is of octahedral geometry, $ {[PtC{l_4}]^{2 - }} $ is of square planar geometry etc.



Note :
Ligands mostly act as donors while sometimes ligand can accept the electrons from metal by pi back bonding in $ {\pi ^*} $ Molecular orbital or vacant d orbital known as pi acid ligand and pi base Metal. Complexes can either be homoleptic or heteroleptic.Homoleptic means Metal surrounded by the same type of ligands and heteroleptic means metal surrounded by 2 or more types of ligands.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




