
What is a tosylate group?
Answer
535.5k+ views
Hint: At the time of doing organic reactions we have to make sure that the other functional groups which are not supposed to be involved in the chemical reaction have to be protected. Tosylate is going to act as a good protecting group for alcohol functional groups.
Complete step-by-step answer:
- In the question it is asked what is a tosylate group.
- Tosylate is anionic moiety of the tosylic acid.
- The structure of tosylic acid is as follows.
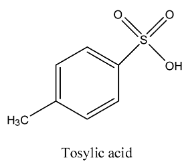
- The structure of tosylate is as follows.
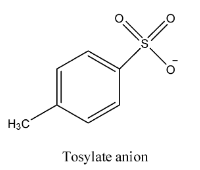
- But in the market it is available in the form of p-toluenesulfonyl chloride.
- The structure of p-toluenesulfonyl chloride is as follows.
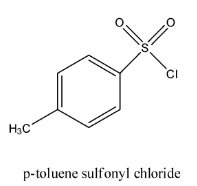
- Alcohols which are present in the organic compounds can be converted into tosylate with the help of tosyl chloride and the chemical reaction is as follows.
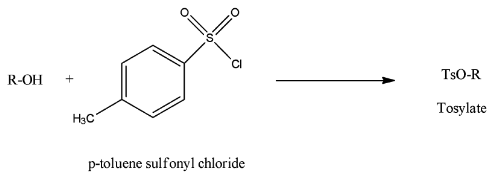
- This is how we can protect the alcohols and later we can remove the tosyl group with the help of an acid.
- Tosylate groups are not only going to act as protecting groups they are good leaving groups.
- After blocking the alcohol with the tosyl group we can convert the tosylate into alkanes with the help of a strong reducing agent.
Note: Tosyl groups have a lot of applications in the organic synthesis field. Tosylates are going to act as a good leaving rather than alcohol. Like tosylates, mesylates (methane sulfonyl chloride) are available and can be used in organic synthesis.
Complete step-by-step answer:
- In the question it is asked what is a tosylate group.
- Tosylate is anionic moiety of the tosylic acid.
- The structure of tosylic acid is as follows.

- The structure of tosylate is as follows.

- But in the market it is available in the form of p-toluenesulfonyl chloride.
- The structure of p-toluenesulfonyl chloride is as follows.

- Alcohols which are present in the organic compounds can be converted into tosylate with the help of tosyl chloride and the chemical reaction is as follows.

- This is how we can protect the alcohols and later we can remove the tosyl group with the help of an acid.
- Tosylate groups are not only going to act as protecting groups they are good leaving groups.
- After blocking the alcohol with the tosyl group we can convert the tosylate into alkanes with the help of a strong reducing agent.
Note: Tosyl groups have a lot of applications in the organic synthesis field. Tosylates are going to act as a good leaving rather than alcohol. Like tosylates, mesylates (methane sulfonyl chloride) are available and can be used in organic synthesis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




