
What is the Bohr Model for Magnesium?
Answer
531k+ views
Hint :We know that by drawing a nucleus with required number of shells. Since the total number of electrons in a magnesium atom is $ 12. $ so start them up in the shells around the nucleus one by one keeping in mind the number of electrons in a shell.
Complete Step By Step Answer:
Neil Bohr proposed an early model of an atom in which central nuclei containing neutrons and protons are surrounded by electrons revolving around them in different shells. In all electrically neutral atoms, the no. of electrons is equal to the number of protons which are certainly equal to the atomic number of the element which makes it different from every other element.
Magnesium $ (Mg) $ is a s block element and has atomic number $ 12. $ It is an extremely important mineral as it is involved in various reactions taking place in our body its electronic configuration is given as; $ [Ne]3{{s}^{2}} $
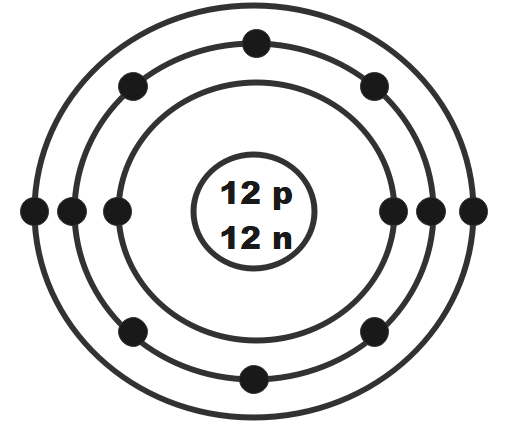
Magnesium has $ 12 $ protons and $ 12 $ electrons. The first electron shell of a Bohr model holds two electrons. The second holds $ 8. $ So far, $ 10, $ of magnesium's $ 12 $ electrons have been used, so only two remain. The remaining two are placed in the third electron shell, which is full when it holds eight electrons.
Therefore, the Bohr Model for Magnesium:
Note :
Note that the electrons in the outermost shell or energy level are helpful in finding the properties of that particular element. Also these electrons are known as valence electrons which play a vital role in reactions. The total no. of valence electrons of magnesium is two.
Complete Step By Step Answer:
Neil Bohr proposed an early model of an atom in which central nuclei containing neutrons and protons are surrounded by electrons revolving around them in different shells. In all electrically neutral atoms, the no. of electrons is equal to the number of protons which are certainly equal to the atomic number of the element which makes it different from every other element.
Magnesium $ (Mg) $ is a s block element and has atomic number $ 12. $ It is an extremely important mineral as it is involved in various reactions taking place in our body its electronic configuration is given as; $ [Ne]3{{s}^{2}} $
Magnesium has $ 12 $ protons and $ 12 $ electrons. The first electron shell of a Bohr model holds two electrons. The second holds $ 8. $ So far, $ 10, $ of magnesium's $ 12 $ electrons have been used, so only two remain. The remaining two are placed in the third electron shell, which is full when it holds eight electrons.
Therefore, the Bohr Model for Magnesium:

Note :
Note that the electrons in the outermost shell or energy level are helpful in finding the properties of that particular element. Also these electrons are known as valence electrons which play a vital role in reactions. The total no. of valence electrons of magnesium is two.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




