
What is the bond polarity of $H_2O$?
Answer
495.6k+ views
Hint: A state or situation of a molecule with positive and negative charges, especially when magnetic or electrical poles are present. Every molecule has a fixed number of electrons that are organised in a shell at specific energy levels. The valence shell electrons are engaged in chemical bonding with other atoms. To achieve stability, atoms tend to adopt the noble gas structure. As a result, we may deduce that chemical bonding is responsible for atom and molecule stability. Chemical bonds can be of many types based on the participation of atoms and the moving of electrons, such as metallic bonds, covalent bonds, and ionic bonds.
Complete answer:
A polar molecule is created when one end of the molecule has a large amount of positive charges and the other end of the molecule contains negative charges, resulting in an electrical dipole. When a molecule or an atom is considered to have a polar bond, the centre of negative charge is on one side and the centre of positive charge is on the other. It will be a polar molecule in its whole.
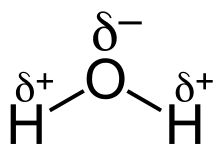
The separation of charge within a bond is referred to as bond polarity. Polar bonds exist between atoms with differing electronegativity. The atom with a larger density of bonding electrons surrounding it has a partial negative charge (${{\delta }^{-}}$). The electron density of the less electronegative atom is reduced, giving it a partial positive charge (${{\delta }^{+}}$). The differing electronegativities of oxygen and hydrogen in water cause charge separation in the O-H bonds. An O-H bond is polar covalent because oxygen is more electronegative than hydrogen. Each O-H bond is polar covalent, with the oxygen atom having a partial negative charge.
A polar covalent bond is represented by the water molecule, written as $H_2O$. The oxygen atom spends more time with electrons than the hydrogen atoms because electrons are shared unequally. The oxygen atom has a partial negative charge because electrons spend more time with it.
Note:
Water is also a polar molecule, which is a result of its structure. A bond dipole moment points from each H to the O due to the difference in electronegativity, making the oxygen partially negative and the hydrogen partially positive. A significant molecular dipole points to the oxygen atom from a location between the two hydrogen atoms. Water molecules agglomerate due to charge differences (the relatively positive areas being attracted to the relatively negative areas). Many of the characteristics of water, such as its solvent qualities, are explained by this attraction, hydrogen bonding.
Complete answer:
A polar molecule is created when one end of the molecule has a large amount of positive charges and the other end of the molecule contains negative charges, resulting in an electrical dipole. When a molecule or an atom is considered to have a polar bond, the centre of negative charge is on one side and the centre of positive charge is on the other. It will be a polar molecule in its whole.
The separation of charge within a bond is referred to as bond polarity. Polar bonds exist between atoms with differing electronegativity. The atom with a larger density of bonding electrons surrounding it has a partial negative charge (${{\delta }^{-}}$). The electron density of the less electronegative atom is reduced, giving it a partial positive charge (${{\delta }^{+}}$). The differing electronegativities of oxygen and hydrogen in water cause charge separation in the O-H bonds. An O-H bond is polar covalent because oxygen is more electronegative than hydrogen. Each O-H bond is polar covalent, with the oxygen atom having a partial negative charge.
A polar covalent bond is represented by the water molecule, written as $H_2O$. The oxygen atom spends more time with electrons than the hydrogen atoms because electrons are shared unequally. The oxygen atom has a partial negative charge because electrons spend more time with it.

Note:
Water is also a polar molecule, which is a result of its structure. A bond dipole moment points from each H to the O due to the difference in electronegativity, making the oxygen partially negative and the hydrogen partially positive. A significant molecular dipole points to the oxygen atom from a location between the two hydrogen atoms. Water molecules agglomerate due to charge differences (the relatively positive areas being attracted to the relatively negative areas). Many of the characteristics of water, such as its solvent qualities, are explained by this attraction, hydrogen bonding.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




