
What is the hybridization of $P{{F}_{5}}$?
Answer
540k+ views
Hint: The central metal atom in $P{{F}_{5}}$is P atom and five F atoms are the outer atoms. The atomic number of P is 15 and it has five valence electrons and the atomic number of F is 9 it has seven valence electrons.
Complete step-by-step answer:So from the lower classes, we are studying many compounds which involve the bonding between various atoms. In this question, we will be discussing the compound formed between phosphorus atoms and fluorine atoms, the type of bonding the atoms, and the hybridization involved.
Now first we have to identify which is the central atom in the compound. The least electronegative atom present in the compound will be the central atom. Since P is less electronegative than F, P is the central atom.
The atomic number of P is 15, it belongs to group 15 or V A, hence it will have 5 valence electrons which can take part in the bonding.
There are five F atoms present to bond with P. The atomic number of F is 9, it belongs to group 17 or VII A, hence there are 7 valence electrons present in F and it needs only one electron to complete its octet.
Now lets us discuss the electronic configuration of P. Since the atomic number of P is 15, there will be 15 electrons in the P atom.
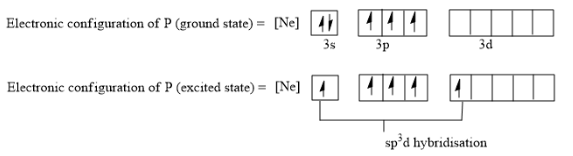
The electronic configuration of P is: $\left[ Ne \right]3{{s}^{2}}3{{p}^{3}}$
Since P has only three sets of unpaired electrons to bond with, but 5 F atoms are present, hence one electron from 3s orbital is excited to 3d orbitals which will make 5 sets of unpaired electrons available for bonding.
One s orbital, three p orbitals, and one d orbital is involved in bonding so the hybridization is $s{{p}^{3}}d$. So a total of $s{{p}^{3}}d$5 hybridized orbitals are formed.
The five $s{{p}^{3}}d$ hybrid orbitals of P bond with five F atoms forming five O-F sigma bonds.
So in the molecule, there are five bond pairs and no lone pairs and the molecule type is $A{{B}_{5}}$.
Note:Since the molecule type is $A{{B}_{5}}$, it will have trigonal bipyramidal geometry. Three F atoms will be positioned in the equatorial position which is arranged ${{120}^{\circ }}$ with each and two F atoms are positioned in the axial position which is arranged at the right angle to the plane.
If we know the number of atoms and lone pairs associated with the central atom, then we can calculate the steric number with which we can identify the hybridization of the molecule.
In this molecule, five F atoms are associated with the central atom and there are no lone pairs, hence the hybridization of the molecule is:
$Steric\,number=atoms+lone\,pairs$
$steric\,number=5+0=5$
The steric number is 5 and the hybridization associated with the steric number 5 is $s{{p}^{3}}d$.
Complete step-by-step answer:So from the lower classes, we are studying many compounds which involve the bonding between various atoms. In this question, we will be discussing the compound formed between phosphorus atoms and fluorine atoms, the type of bonding the atoms, and the hybridization involved.
Now first we have to identify which is the central atom in the compound. The least electronegative atom present in the compound will be the central atom. Since P is less electronegative than F, P is the central atom.
The atomic number of P is 15, it belongs to group 15 or V A, hence it will have 5 valence electrons which can take part in the bonding.
There are five F atoms present to bond with P. The atomic number of F is 9, it belongs to group 17 or VII A, hence there are 7 valence electrons present in F and it needs only one electron to complete its octet.
Now lets us discuss the electronic configuration of P. Since the atomic number of P is 15, there will be 15 electrons in the P atom.
The electronic configuration of P is: $\left[ Ne \right]3{{s}^{2}}3{{p}^{3}}$
Since P has only three sets of unpaired electrons to bond with, but 5 F atoms are present, hence one electron from 3s orbital is excited to 3d orbitals which will make 5 sets of unpaired electrons available for bonding.
One s orbital, three p orbitals, and one d orbital is involved in bonding so the hybridization is $s{{p}^{3}}d$. So a total of $s{{p}^{3}}d$5 hybridized orbitals are formed.

The five $s{{p}^{3}}d$ hybrid orbitals of P bond with five F atoms forming five O-F sigma bonds.
So in the molecule, there are five bond pairs and no lone pairs and the molecule type is $A{{B}_{5}}$.
Note:Since the molecule type is $A{{B}_{5}}$, it will have trigonal bipyramidal geometry. Three F atoms will be positioned in the equatorial position which is arranged ${{120}^{\circ }}$ with each and two F atoms are positioned in the axial position which is arranged at the right angle to the plane.
If we know the number of atoms and lone pairs associated with the central atom, then we can calculate the steric number with which we can identify the hybridization of the molecule.
In this molecule, five F atoms are associated with the central atom and there are no lone pairs, hence the hybridization of the molecule is:
$Steric\,number=atoms+lone\,pairs$
$steric\,number=5+0=5$
The steric number is 5 and the hybridization associated with the steric number 5 is $s{{p}^{3}}d$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




