
What is the IUPAC name of ${(CC{l_3})_3}CCl$?
Answer
597.6k+ views
Hint: The trivial name of the given compound is perchloric isobutane. It is also known as tris-(trichloromethyl) chloromethane. The molecular formula of the compound is ${C_4}C{l_{10}}$ and molecular weight $402.54$ gram. The structure of the compound ${(CC{l_3})_3}CCl$ is given below;
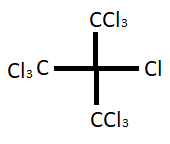
Complete step by step solution:
So let's start the IUPAC naming step by step of the given compound ${(CC{l_3})_3}CCl$ or perchloric isobutane. We know that while writing IUPAC the name of any compound halogens never takes precedence, so here in this compound there is no other functional group except halogen (chlorine) so we will find out the longest continuous carbon chain. In this compound the longest chain contains three carbon atoms so the root word would be prop and there is no double or triple bond so we will use primary suffix ane. We can number the carbon atoms of principal chain as follows;
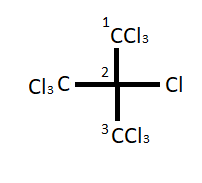
We can see in the compound that on the second carbon atom there is a trichloromethyl substituent. On the first and third carbon the compound has six chloro substituents. Together with the extra chloro substituent at second carbon, there are seven chloro substituents.
So the IUPAC name of the compound will be ;$2 - \left( {Trichloromethyl} \right) - 1,1,1,2,3,3,3 - heptafluoropropane$, Here number $1,1,1,2,3,3,3$ refers the position of chloro substituents in the carbon chain.
Note: So we have approached the naming of the given compound by counting the carbon atoms in the main chain and then we name the primary suffix and prefix. If all the hydrogen atoms of isobutane structure are replaced with chlorine then the given compound is formed therefore it is called perchloric isobutane.

Complete step by step solution:
So let's start the IUPAC naming step by step of the given compound ${(CC{l_3})_3}CCl$ or perchloric isobutane. We know that while writing IUPAC the name of any compound halogens never takes precedence, so here in this compound there is no other functional group except halogen (chlorine) so we will find out the longest continuous carbon chain. In this compound the longest chain contains three carbon atoms so the root word would be prop and there is no double or triple bond so we will use primary suffix ane. We can number the carbon atoms of principal chain as follows;

We can see in the compound that on the second carbon atom there is a trichloromethyl substituent. On the first and third carbon the compound has six chloro substituents. Together with the extra chloro substituent at second carbon, there are seven chloro substituents.
So the IUPAC name of the compound will be ;$2 - \left( {Trichloromethyl} \right) - 1,1,1,2,3,3,3 - heptafluoropropane$, Here number $1,1,1,2,3,3,3$ refers the position of chloro substituents in the carbon chain.
Note: So we have approached the naming of the given compound by counting the carbon atoms in the main chain and then we name the primary suffix and prefix. If all the hydrogen atoms of isobutane structure are replaced with chlorine then the given compound is formed therefore it is called perchloric isobutane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




