
What is water gas?
Answer
594k+ views
Hint: Water gas is a gas produced due to the reaction of carbon and water. So, we must know the gases produced by the reaction.
Step-by-step answer:
Passing of steam over red hot coke (a form of carbon) produces water gas which is a combination of two gases, namely, hydrogen and carbon dioxide. The production of water gas is an endothermic reaction, so, to keep the reaction going, heat must be supplied continuously.
Now, we write the reaction of formation of water gas.
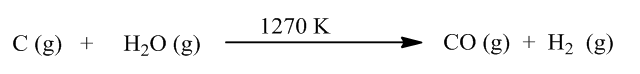
From the above reaction, we can see that on the reaction of water and carbon, carbon monoxide, and hydrogen gas are produced. So, water gas is a mixture of carbon monoxide and hydrogen gas. Water-gas can be prepared from sawdust, sewage, newspaper, and scrap wood. Water gas has many uses, such as it is used for methanol and other hydrocarbon production, it is used as fuel in industries, it is also used for the manufacture of ammonia, etc.
Additional Information:
Synthesis gas is commonly termed as Syngas. This gas is composed of hydrogen, carbon monoxide, and many times carbon dioxide gas also and the gas is a product of gasification of fuel containing carbon. From syngas, water is produced. The production of water gas includes the passing of steam over warm hydrocarbons. The reaction between hydrocarbon and steam gives syngas. By reducing the level of carbon dioxide and increasing hydrogen in the syngas, water gas is produced. There are two types of water gas, Carburetted water gas and semi water gas.
The chemical reaction for formation of syngas from natural gas by reaction with steam is,
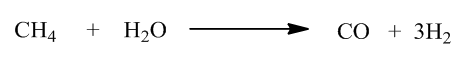
Note: The composition of syngas is carbon monoxide, hydrogen and carbon dioxide while the composition of water gas is carbon monoxide and hydrogen gas. The difference in the composition of both the gas is water gas has no carbon dioxide but syngas composed of carbon dioxide.
Step-by-step answer:
Passing of steam over red hot coke (a form of carbon) produces water gas which is a combination of two gases, namely, hydrogen and carbon dioxide. The production of water gas is an endothermic reaction, so, to keep the reaction going, heat must be supplied continuously.
Now, we write the reaction of formation of water gas.

From the above reaction, we can see that on the reaction of water and carbon, carbon monoxide, and hydrogen gas are produced. So, water gas is a mixture of carbon monoxide and hydrogen gas. Water-gas can be prepared from sawdust, sewage, newspaper, and scrap wood. Water gas has many uses, such as it is used for methanol and other hydrocarbon production, it is used as fuel in industries, it is also used for the manufacture of ammonia, etc.
Additional Information:
Synthesis gas is commonly termed as Syngas. This gas is composed of hydrogen, carbon monoxide, and many times carbon dioxide gas also and the gas is a product of gasification of fuel containing carbon. From syngas, water is produced. The production of water gas includes the passing of steam over warm hydrocarbons. The reaction between hydrocarbon and steam gives syngas. By reducing the level of carbon dioxide and increasing hydrogen in the syngas, water gas is produced. There are two types of water gas, Carburetted water gas and semi water gas.
The chemical reaction for formation of syngas from natural gas by reaction with steam is,

Note: The composition of syngas is carbon monoxide, hydrogen and carbon dioxide while the composition of water gas is carbon monoxide and hydrogen gas. The difference in the composition of both the gas is water gas has no carbon dioxide but syngas composed of carbon dioxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




