
What will be the product T?
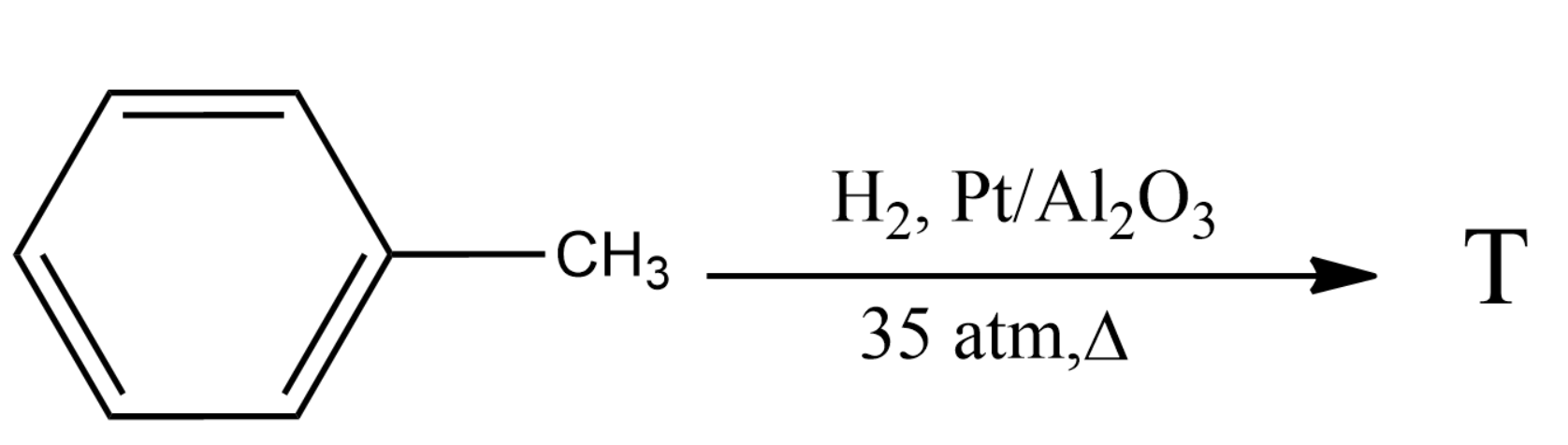
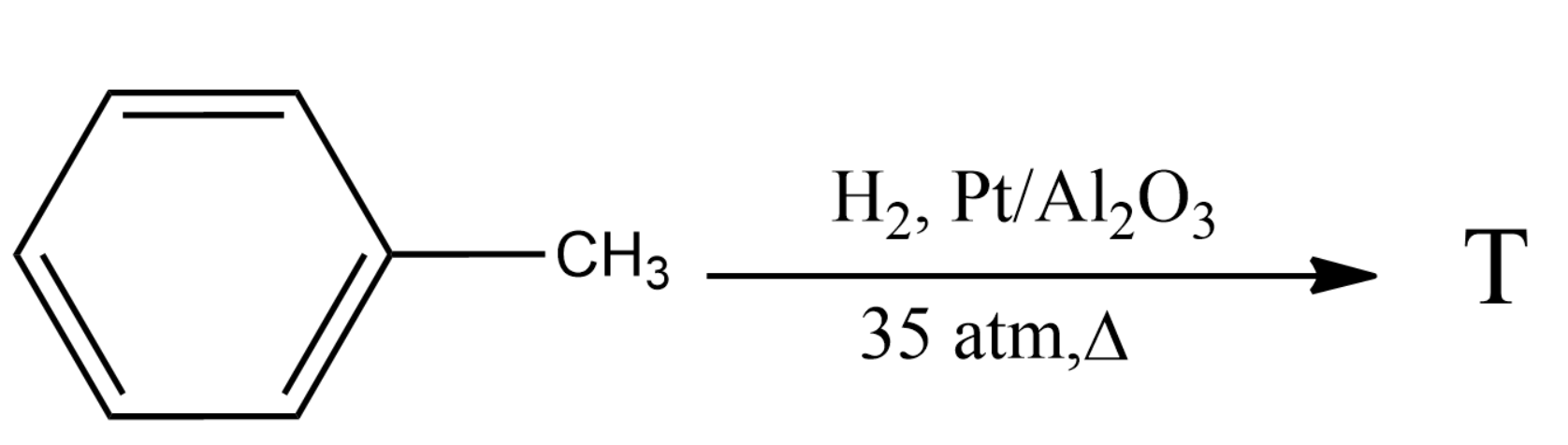
Answer
492.9k+ views
Hint: Transition metals including Ni, Pt, Ru have been studied in hydrogenation of toluene to methylcyclohexane. The toluene will get adsorbed on the catalyst's surface and can be hydrogenated by hydrogen adsorbed through different mechanisms. Hydrogen gets adsorbed on the conventional metal active sites and the spillover hydrogen will migrate from the metal sites to the acidic sites.
Complete answer:
In some extreme cases of extreme hydrogenation where the benzene ring will lose its aromaticity to form a cyclohexane ring which is non-aromatic.
The reaction is done in the presence of hydrogen and a catalyst like platinum. The platinum will provide a good surface for the reactants to adsorb and thus react effectively.
High-pressure facilitates the reaction to enter a thermodynamically controlled regime with an excellent catalytic performance. Strong interplay between the kinetics and thermodynamics determines the catalytic performance.
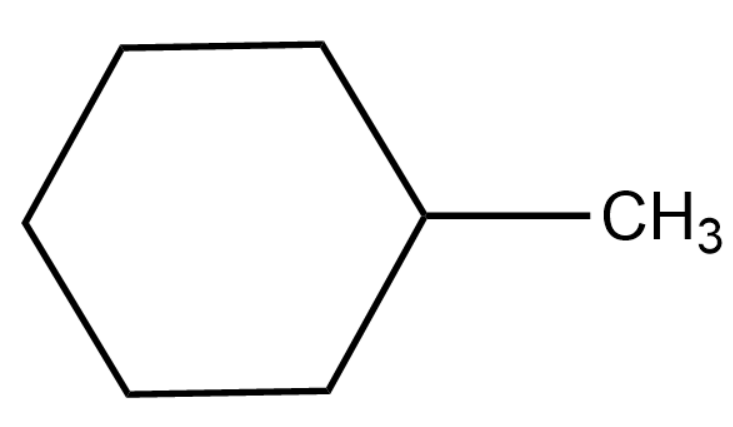
Thus we will obtain methyl cyclohexane as the product of the given reaction.
Thus the product T is
Note:
Hydrogenation is a chemical reaction between molecular hydrogen and any other compounds and elements. Hydrogenation is used in many important applications such as the food industry, petrochemical industry and the pharmaceutical manufacturing industry.
Benzene does not undergo the same reactions that all other alkenes do. This is due to its aromatic stability. Aromatic molecules will have all ring atoms in the same plane to allow delocalization of the pi electrons. This gives them more stability and usually a reaction doesn’t proceed in the direction where this aromatic nature is lost.
Complete answer:
In some extreme cases of extreme hydrogenation where the benzene ring will lose its aromaticity to form a cyclohexane ring which is non-aromatic.
The reaction is done in the presence of hydrogen and a catalyst like platinum. The platinum will provide a good surface for the reactants to adsorb and thus react effectively.
High-pressure facilitates the reaction to enter a thermodynamically controlled regime with an excellent catalytic performance. Strong interplay between the kinetics and thermodynamics determines the catalytic performance.
Thus we will obtain methyl cyclohexane as the product of the given reaction.
Thus the product T is

Note:
Hydrogenation is a chemical reaction between molecular hydrogen and any other compounds and elements. Hydrogenation is used in many important applications such as the food industry, petrochemical industry and the pharmaceutical manufacturing industry.
Benzene does not undergo the same reactions that all other alkenes do. This is due to its aromatic stability. Aromatic molecules will have all ring atoms in the same plane to allow delocalization of the pi electrons. This gives them more stability and usually a reaction doesn’t proceed in the direction where this aromatic nature is lost.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




