
Which alcohol of molecular formula \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}\text{OH}\] cannot be obtained by the reduction of carbonyl compound?
A. \[2-\text{methylpropan}-1-\text{ol}\]
B. \[2-\text{methylpropan}-2-\text{ol}\]
C. Butanol
D. \[\text{Butan}-2-\text{ol}\]
Answer
587.7k+ views
Hint: At first, we need to construct all the structures of the possible alcohols from \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}\text{OH}\]. Then we can examine which of them cannot be obtained from the reduction of carbonyl compounds.
Complete step by step answer:
Here is the structure of \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}\text{OH}\], which is a primary alcohol with 4 carbon in their structure:
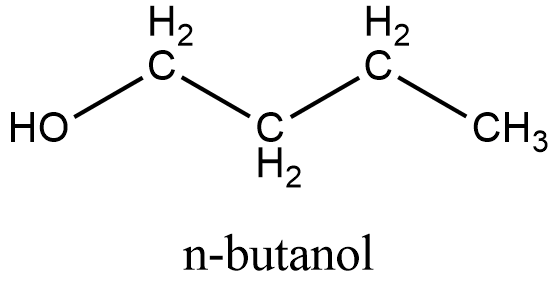
Here are following structures of the options mentioned in the question:
The structure of \[2-\text{methylpropan}-1-\text{ol}\]:
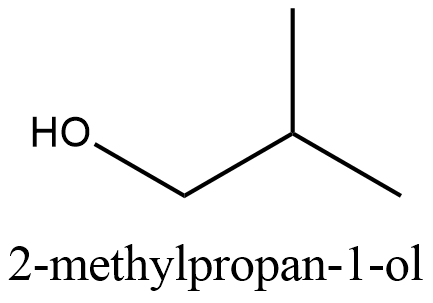
From the structure we can see that it cannot be obtained from the primary alcohol which is mentioned in the question. So this is the correct answer.
The structure of \[2-\text{methylpropan}-2-\text{ol}\]:
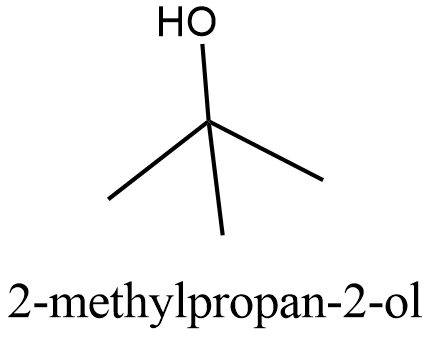
From the structure we can see that it can be obtained from the primary alcohol which is mentioned in the question. So this is not the correct answer.
The structure of Butanol:
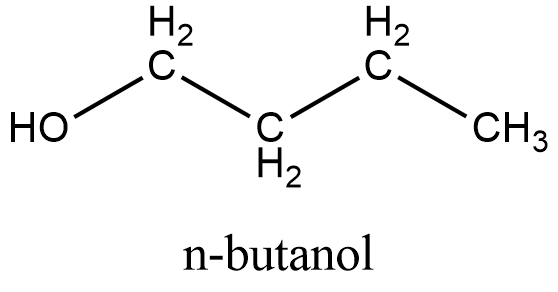
From the structure we can see that it can be obtained from the primary alcohol which is mentioned in the question. So this is not the correct answer.
The structure of \[\text{Butan}-2-\text{ol}\]:
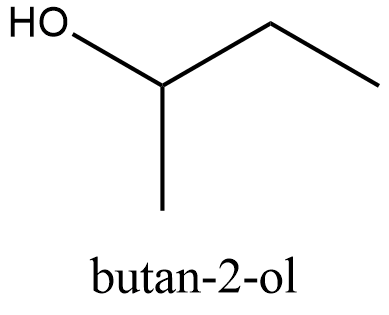
From the structure we can see that it can be obtained from the primary alcohol which is mentioned in the question. So this is not the correct answer.
So as we can see that out of the mentioned options only \[2-\text{methylpropan}-2-\text{ol}\] is not obtained by the reduction of carbonyl compounds.
Therefore, the correct answer to this question is \[2-\text{methylpropan}-2-\text{ol}\].
So, the correct answer is “Option B”.
Additional Information:
Here are some properties of carbonyl compounds, which should be known:
1. These are to be polar in nature. They exhibit both positive and negative charge in slight form.
2. These compounds are reported to be insoluble in water but sometimes they dissolve other forms of polar molecules.
3. These are known to be reactive compounds.
Note: By carbonyl compound, we mean a functional group composed of a carbon atom double bonded to an oxygen atom. The functional group is represented as \[\text{C=O}\].
\[2-\text{methylpropan}-2-\text{ol}\]can be obtained from ethanol. Ethanol has a formula of \[{{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}\].
Complete step by step answer:
Here is the structure of \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}\text{OH}\], which is a primary alcohol with 4 carbon in their structure:

Here are following structures of the options mentioned in the question:
The structure of \[2-\text{methylpropan}-1-\text{ol}\]:

From the structure we can see that it cannot be obtained from the primary alcohol which is mentioned in the question. So this is the correct answer.
The structure of \[2-\text{methylpropan}-2-\text{ol}\]:
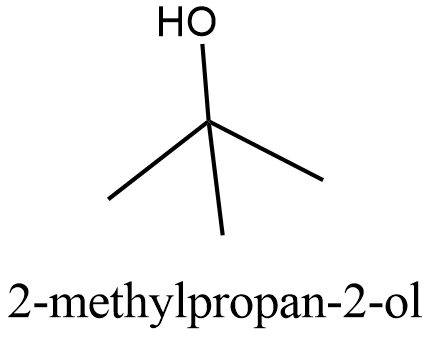
From the structure we can see that it can be obtained from the primary alcohol which is mentioned in the question. So this is not the correct answer.
The structure of Butanol:

From the structure we can see that it can be obtained from the primary alcohol which is mentioned in the question. So this is not the correct answer.
The structure of \[\text{Butan}-2-\text{ol}\]:

From the structure we can see that it can be obtained from the primary alcohol which is mentioned in the question. So this is not the correct answer.
So as we can see that out of the mentioned options only \[2-\text{methylpropan}-2-\text{ol}\] is not obtained by the reduction of carbonyl compounds.
Therefore, the correct answer to this question is \[2-\text{methylpropan}-2-\text{ol}\].
So, the correct answer is “Option B”.
Additional Information:
Here are some properties of carbonyl compounds, which should be known:
1. These are to be polar in nature. They exhibit both positive and negative charge in slight form.
2. These compounds are reported to be insoluble in water but sometimes they dissolve other forms of polar molecules.
3. These are known to be reactive compounds.
Note: By carbonyl compound, we mean a functional group composed of a carbon atom double bonded to an oxygen atom. The functional group is represented as \[\text{C=O}\].
\[2-\text{methylpropan}-2-\text{ol}\]can be obtained from ethanol. Ethanol has a formula of \[{{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




