
Which alkyl halide from the following pairs would you expect to react more rapidly by an \[{S_N}2\] mechanism? Explain your answer.

Answer
565.2k+ views
Hint:. \[{S_N}2\] mechanism is a bimolecular Nucleophilic substitution reaction. It is a second order reaction whose rate depends on two species namely the nucleophile and the substrate. The reaction will take place in one step.
Complete step by step answer:
- \[{S_N}2\] mechanism stands for the bimolecular nucleophilic substitution reaction.
-The rate of \[{S_N}2\] reaction depends on the concentration of two species namely nucleophile and substrate.
-This is a second order reaction. It is a one-step mechanism.
- In \[{S_N}2\] mechanism the nucleophile attacks the substrate from the backside.
- A transition state containing both nucleophile and leaving group will be formed. In this transition state, the bond between nucleophile and substrate is partially formed and bond between leaving and the substrate is partially broken.
- Nucleophile approaches the substrate exactly at \[180^\circ \] to the position of the leaving group.
- Reactivity of alkyl halide in \[{S_N}2\] mechanisms depend on the following order:
\[{1^\circ } > {2^\circ } > {3^\circ }\]
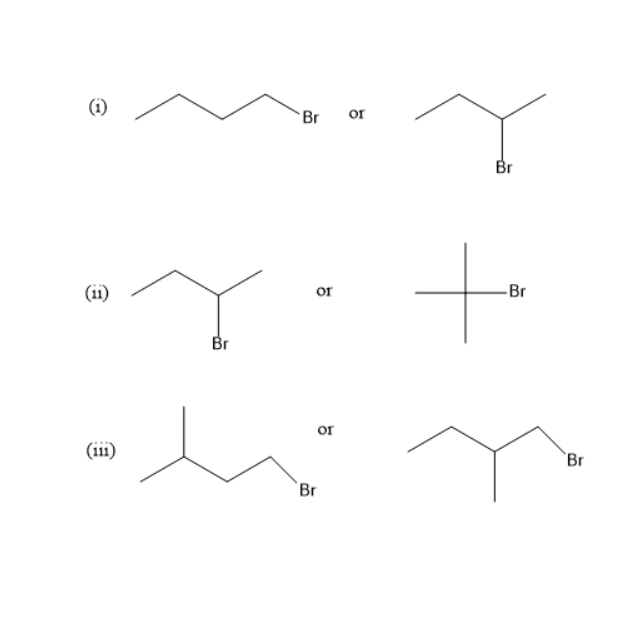
(i) 1-Bromobutane is a primary alkyl halide while 2-Bromobutane is a secondary alkyl halide. The nucleophile which approaches \[{2^\circ }\] alkyl halide will be more hindered compared to \[{1^\circ }\]alkyl halide. Therefore, 1-Bromobutane is more reactive in \[{S_N}2\] mechanisms than 2-Bromobutane
(ii) 2-Bromobutane is a secondary alkyl halide while 2-Bromo-2-methylpropane is a tertiary alkyl halide. Therefore, 2-Bromobutane is more reactive in \[{S_N}2\] mechanisms than 2-Bromo-2-methylpropane. The reason is 2-Bromo-2-methylpropane being alkyl halide is a bulky group and is very much hindered. Therefore, 2-Bromobutane is more reactive.
(iii) Both 1-Bromo-3-methylbutane and1-Bromo-2-methylbutane are \[{1^\circ }\] alkyl halide. In 1-Bromo-3-methylbutane, methyl group is present far away from bromine, so reactivity of 1-Bromo-3-methylbutane is greater compared to1-Bromo-2-methylbutane.
Therefore,
- In the reaction (i), 1-Bromobutane is more reactive.
- In the reaction (ii), 2-Bromobutane is more reactive.
- In the reaction (iii), 1-Bromo-3-methylbutane is more reactive.
Additional information:
Note: - Reactivity of different alkyl halides in \[{S_N}2\] mechanism is given the following order:
\[{1^\circ } > {2^\circ } > {3^\circ }\]
- Reactivity of different alkyl halides in \[{S_N}1\] mechanism is given the following order
\[3^\circ > 2^\circ > 1^\circ \]
Complete step by step answer:
- \[{S_N}2\] mechanism stands for the bimolecular nucleophilic substitution reaction.
-The rate of \[{S_N}2\] reaction depends on the concentration of two species namely nucleophile and substrate.
-This is a second order reaction. It is a one-step mechanism.
- In \[{S_N}2\] mechanism the nucleophile attacks the substrate from the backside.
- A transition state containing both nucleophile and leaving group will be formed. In this transition state, the bond between nucleophile and substrate is partially formed and bond between leaving and the substrate is partially broken.
- Nucleophile approaches the substrate exactly at \[180^\circ \] to the position of the leaving group.
- Reactivity of alkyl halide in \[{S_N}2\] mechanisms depend on the following order:
\[{1^\circ } > {2^\circ } > {3^\circ }\]
(i) 1-Bromobutane is a primary alkyl halide while 2-Bromobutane is a secondary alkyl halide. The nucleophile which approaches \[{2^\circ }\] alkyl halide will be more hindered compared to \[{1^\circ }\]alkyl halide. Therefore, 1-Bromobutane is more reactive in \[{S_N}2\] mechanisms than 2-Bromobutane
(ii) 2-Bromobutane is a secondary alkyl halide while 2-Bromo-2-methylpropane is a tertiary alkyl halide. Therefore, 2-Bromobutane is more reactive in \[{S_N}2\] mechanisms than 2-Bromo-2-methylpropane. The reason is 2-Bromo-2-methylpropane being alkyl halide is a bulky group and is very much hindered. Therefore, 2-Bromobutane is more reactive.
(iii) Both 1-Bromo-3-methylbutane and1-Bromo-2-methylbutane are \[{1^\circ }\] alkyl halide. In 1-Bromo-3-methylbutane, methyl group is present far away from bromine, so reactivity of 1-Bromo-3-methylbutane is greater compared to1-Bromo-2-methylbutane.
Therefore,
- In the reaction (i), 1-Bromobutane is more reactive.
- In the reaction (ii), 2-Bromobutane is more reactive.
- In the reaction (iii), 1-Bromo-3-methylbutane is more reactive.
Additional information:
| \[{S_N}2\] mechanism | \[{S_N}1\] mechanism |
| One-step process | Two-step process |
| Rate depends on two species i.e. nucleophile and substrate. | Rate depends on only one species i.e. substrate. |
| Reaction is favored by non-polar solvents. | Reaction is favored by polar solvents. |
| It is bimolecular. | It is unimolecular. |
| It is a second order reaction. | It is a first order reaction. |
Note: - Reactivity of different alkyl halides in \[{S_N}2\] mechanism is given the following order:
\[{1^\circ } > {2^\circ } > {3^\circ }\]
- Reactivity of different alkyl halides in \[{S_N}1\] mechanism is given the following order
\[3^\circ > 2^\circ > 1^\circ \]
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




