
Which among the following show tautomerism?
A.Alcohols
B.Phenols
C.Ethers
D.Anisole
Answer
521.1k+ views
Hint: Isomerism: When two or more compounds have the same molecular formula but differ in structural formula, are known as isomers and the phenomenon is termed as isomerism. There are majorly two types of isomerism i.e., structural isomerism and stereoisomerism.
Complete answer:
Tautomerism: It is a process where a single chemical compound exhibits more than one interconvertible structure that generally differs in terms of relative positions of hydrogen atoms. In other words, we can say that the compounds showing tautomerism possess dynamic equilibrium.
Alcohol: In alcohols, generally the compounds are saturated compounds of type \[R - OH\]. Due to no unsaturation in any bond, there will be no movement of electrons or protons in the structure. Hence it does not show tautomerism.
Phenol: The molecular formula of phenol is \[{C_6}{H_5} - OH\] and its structural formula is as follows:
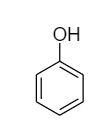
Due to the presence of conjugated double bonds, it can show tautomerism by changing the position of protons present in the hydroxyl group. The interconvertible structures that show tautomerism are shown below:
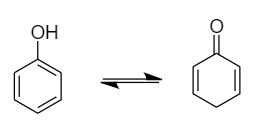
Therefore, the phenols can show tautomerism.
Ether: The structural formula of ether can be represented as \[R - O - R\], there is no possibility of transfer of protons and in general, the compound is saturated. Therefore, it does not show tautomerism.
Anisole: The molecular formula of anisole is \[{C_6}{H_5} - OC{H_3}\] and its structural formula is as follows:
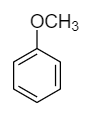
Although the double bond is present in the structure, there is no proton present with the oxygen atom. So, no proton shift takes place and therefore it does not show tautomerism.
Note:
In phenol, the first structure is more stable than the second structure because it consists of properties like aromaticity and resonance which make it highly stable. Therefore, phenol majorly exists as follows:
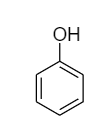
Complete answer:
Tautomerism: It is a process where a single chemical compound exhibits more than one interconvertible structure that generally differs in terms of relative positions of hydrogen atoms. In other words, we can say that the compounds showing tautomerism possess dynamic equilibrium.
Alcohol: In alcohols, generally the compounds are saturated compounds of type \[R - OH\]. Due to no unsaturation in any bond, there will be no movement of electrons or protons in the structure. Hence it does not show tautomerism.
Phenol: The molecular formula of phenol is \[{C_6}{H_5} - OH\] and its structural formula is as follows:

Due to the presence of conjugated double bonds, it can show tautomerism by changing the position of protons present in the hydroxyl group. The interconvertible structures that show tautomerism are shown below:

Therefore, the phenols can show tautomerism.
Ether: The structural formula of ether can be represented as \[R - O - R\], there is no possibility of transfer of protons and in general, the compound is saturated. Therefore, it does not show tautomerism.
Anisole: The molecular formula of anisole is \[{C_6}{H_5} - OC{H_3}\] and its structural formula is as follows:

Although the double bond is present in the structure, there is no proton present with the oxygen atom. So, no proton shift takes place and therefore it does not show tautomerism.
Note:
In phenol, the first structure is more stable than the second structure because it consists of properties like aromaticity and resonance which make it highly stable. Therefore, phenol majorly exists as follows:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




