
Which are the correct IUPAC names of the following compounds?
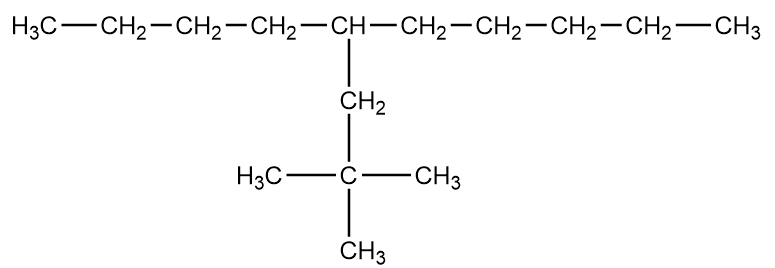
A. 5-(2’,2’-dimethylpropyl) decane
B. 4-butyl-2,2-dimethyl nonane
C. 2,2-dimethyl-4-pentyl octane
D. 5-neopentyl decane

Answer
512.1k+ views
Hint :The systematic approach to IUPAC nomenclature relies on two basic features, first it should indicate the characteristic lattice of chain and ring in which carbon atoms are bonded together in a compound and second is to identifying and locating the functional groups (if any) with in the given compound.
Complete Step By Step Answer:
According to IUPAC, the standard rules for naming an alkane are as follows:
1. Identify the longest continuous carbon chain in the compound and name it accordingly.
2. Find the functional groups or substituent groups attached to the chain and name the groups as per IUPAC rules.
3. Numbering of parent or base carbon chains should be done in such a manner that the substituent groups get minimum allocation value.
4. Designate the location of each substituent group present in the chain by a proper number and name.
5. Arrange the name of functional group or substituent groups in alphabetical order followed by the name of parent chain.
6. The prefixes like di, tri, tera, etc are used when more than one substituent groups of the same kind are present in the carbon chain.
Therefore, for the given compound, the longest carbon chain i.e., the parent chain will be as follows:
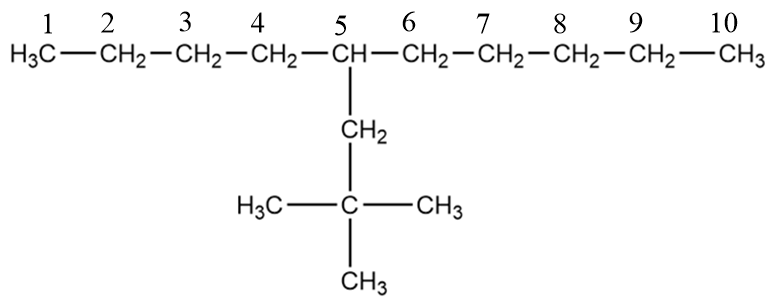
The number of carbon atoms present in the base chain $ = 10 $
So, the name of the base chain is decane.
Now, let us look at the name of substituent group present in the compound:
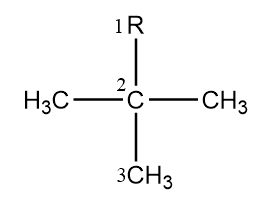
We can name this group in two ways which are as follows:
Common name- neopentyl
IUPAC name- 2,2-dimethylpropyl
Hence, the correct IUPAC names for the given compounds are as follows:
5-(2’,2’-dimethylpropyl) decane
5-neopentyl decane
Thus, options (A) and (D) are the correct answers.
Note :
It is important to note that in the name of substituent groups, numbers are written along with an apostrophe (’) sign to differentiate the groups of substituent carbon atoms from the groups of parent chain. Also, if two or more longest chains are present within a structure, then the chain with largest substituent groups is chosen.
Complete Step By Step Answer:
According to IUPAC, the standard rules for naming an alkane are as follows:
1. Identify the longest continuous carbon chain in the compound and name it accordingly.
2. Find the functional groups or substituent groups attached to the chain and name the groups as per IUPAC rules.
3. Numbering of parent or base carbon chains should be done in such a manner that the substituent groups get minimum allocation value.
4. Designate the location of each substituent group present in the chain by a proper number and name.
5. Arrange the name of functional group or substituent groups in alphabetical order followed by the name of parent chain.
6. The prefixes like di, tri, tera, etc are used when more than one substituent groups of the same kind are present in the carbon chain.
Therefore, for the given compound, the longest carbon chain i.e., the parent chain will be as follows:

The number of carbon atoms present in the base chain $ = 10 $
So, the name of the base chain is decane.
Now, let us look at the name of substituent group present in the compound:

We can name this group in two ways which are as follows:
Common name- neopentyl
IUPAC name- 2,2-dimethylpropyl
Hence, the correct IUPAC names for the given compounds are as follows:
5-(2’,2’-dimethylpropyl) decane
5-neopentyl decane
Thus, options (A) and (D) are the correct answers.
Note :
It is important to note that in the name of substituent groups, numbers are written along with an apostrophe (’) sign to differentiate the groups of substituent carbon atoms from the groups of parent chain. Also, if two or more longest chains are present within a structure, then the chain with largest substituent groups is chosen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




