
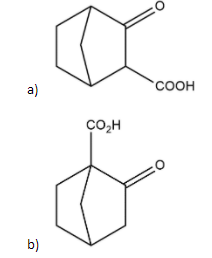
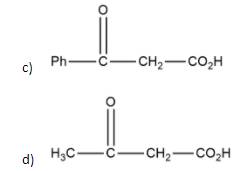
Which \[\beta \]- keto acid shown will not undergo decarboxylation?


Answer
582.3k+ views
Hint: Keto acids are also called oxo acids. These are organic compounds that contain a carboxylic acid group and a ketone group. There are 3 types of keto acids alpha, beta and gamma keto acids.
Complete step by step answer:
Compounds which contain carboxylic \[COOH\]and ketone group \[C=O\]present in it are known as keto acids or oxo acids. On the basis of attachment of ketone groups these are divided into 3 parts.
\[\alpha \]- keto acid in which ketone group is present at alpha carbon atom
\[\beta \]- keto acid in which ketone group is present at beta carbon atom
γ - keto acid in which ketone group is present at gamma carbon atom which can be shown as:
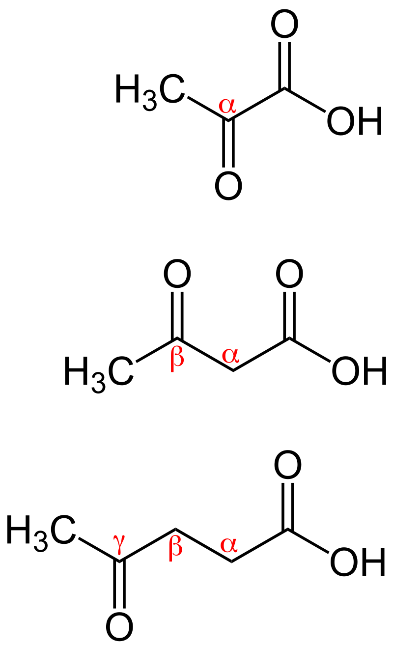
Decarboxylation takes place when carboxylic acids containing a carbonyl group two bonds away (on the \[\beta \]carbon) are heated and carbon dioxide is lost during this process.
In the case of compound b, \[\alpha \]\[\beta \]−keto acid having it's \[\alpha \]carbon at bridged head so it can't undergo tautomerization and it has a fixed hybridisation of \[s{{p}^{3}}\] and can't undergo decarboxylation while on the other compounds \[\beta \] position is free so decarboxylation can be occur easily.
Thus, option b is right.
Note: Decarboxylation is the process in which loss of carbon dioxide is there in this process of tautomerization. Tautomerism is the ability of certain organic compounds to react in isomeric structures that differ from each other in the position of a hydrogen atom and a double bond.
Complete step by step answer:
Compounds which contain carboxylic \[COOH\]and ketone group \[C=O\]present in it are known as keto acids or oxo acids. On the basis of attachment of ketone groups these are divided into 3 parts.
\[\alpha \]- keto acid in which ketone group is present at alpha carbon atom
\[\beta \]- keto acid in which ketone group is present at beta carbon atom
γ - keto acid in which ketone group is present at gamma carbon atom which can be shown as:

Decarboxylation takes place when carboxylic acids containing a carbonyl group two bonds away (on the \[\beta \]carbon) are heated and carbon dioxide is lost during this process.
In the case of compound b, \[\alpha \]\[\beta \]−keto acid having it's \[\alpha \]carbon at bridged head so it can't undergo tautomerization and it has a fixed hybridisation of \[s{{p}^{3}}\] and can't undergo decarboxylation while on the other compounds \[\beta \] position is free so decarboxylation can be occur easily.
Thus, option b is right.
Note: Decarboxylation is the process in which loss of carbon dioxide is there in this process of tautomerization. Tautomerism is the ability of certain organic compounds to react in isomeric structures that differ from each other in the position of a hydrogen atom and a double bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




