
Which combination will give the strongest ionic bond?
A.$N{a^ + }$ and $C{l^ - }$
B.$N{a^ + }$ and ${O_2}^ - $
C.$M{g^{ + 2}}$ and $C{l^ - }$
D.$M{g^{ + 2}}$ and ${O_2}^ - $
Answer
508.5k+ views
Hint: Atoms form different chemical bonds between the constituent particles which is a type of an attractive force which holds them together.
When a complete transfer of one or more valence electrons from an atom to another atom takes place, it will result in the formation of ionic bonds.
Complete answer:
There are some ideal conditions which must be satisfied to form a strong ionic bond.
a.As the value of lattice energy increases the strength of ionic bonds also increases.
b.As the size of ions decreases, the internuclear distance between two ions also decreases and results in formation of strong ionic bonds.
c.Strength of ionic bond is directly proportional to the magnitude of charge present over the particular ion.
Keeping all these rules in mind we took different ions given to determine their ionic bond strength.
According to the general properties of the periodic table when we move from left to right the size of an atom increases because the number of electrons in the valence shell increases.
Since, sodium is member of group $\left( {IA} \right)$ and magnesium in group $\left( {IIA} \right)$ . size of $M{g^{ + 2}}$ is greater than $N{a^ + }$. But due to removal of two electrons from $M{g^{ + 2}}$its ionic radius becomes smaller than $N{a^ + }$.
Hence, $M{g^{ + 2}} > N{a^ + }$.
When we move top to bottom in a periodic table, the size of the atom increases hence, the size of ${O_2}^ - $ is smaller than $C{l^ - }$.
From the given options we see that $M{g^{ + 2}}$ has the maximum magnitude of charge that is $\left( { + 2} \right)$ while rest of the ions contain $\left( { \pm 1} \right)$ charge .
On the basis of these points we see that $M{g^{ + 2}}$and${O_2}^ - $ has the maximum magnitude of charge and has the smallest ionic size .
Combination of $M{g^{ + 2}}$and${O_2}^ - $ has the maximum lattice energy.
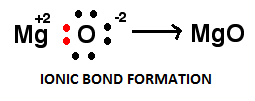
Therefore, $M{g^{ + 2}}$ and ${O_2}^ - $ forms the strongest ionic bond.
Hence option D is correct .
Note:
Only valence electrons of an atom take part in ionic bond formation.
Lattice energy is defined as the total amount of energy evolved when free ions of an atom combine together to form one mole of crystal.
When a complete transfer of one or more valence electrons from an atom to another atom takes place, it will result in the formation of ionic bonds.
Complete answer:
There are some ideal conditions which must be satisfied to form a strong ionic bond.
a.As the value of lattice energy increases the strength of ionic bonds also increases.
b.As the size of ions decreases, the internuclear distance between two ions also decreases and results in formation of strong ionic bonds.
c.Strength of ionic bond is directly proportional to the magnitude of charge present over the particular ion.
Keeping all these rules in mind we took different ions given to determine their ionic bond strength.
According to the general properties of the periodic table when we move from left to right the size of an atom increases because the number of electrons in the valence shell increases.
Since, sodium is member of group $\left( {IA} \right)$ and magnesium in group $\left( {IIA} \right)$ . size of $M{g^{ + 2}}$ is greater than $N{a^ + }$. But due to removal of two electrons from $M{g^{ + 2}}$its ionic radius becomes smaller than $N{a^ + }$.
Hence, $M{g^{ + 2}} > N{a^ + }$.
When we move top to bottom in a periodic table, the size of the atom increases hence, the size of ${O_2}^ - $ is smaller than $C{l^ - }$.
From the given options we see that $M{g^{ + 2}}$ has the maximum magnitude of charge that is $\left( { + 2} \right)$ while rest of the ions contain $\left( { \pm 1} \right)$ charge .
On the basis of these points we see that $M{g^{ + 2}}$and${O_2}^ - $ has the maximum magnitude of charge and has the smallest ionic size .
Combination of $M{g^{ + 2}}$and${O_2}^ - $ has the maximum lattice energy.

Therefore, $M{g^{ + 2}}$ and ${O_2}^ - $ forms the strongest ionic bond.
Hence option D is correct .
Note:
Only valence electrons of an atom take part in ionic bond formation.
Lattice energy is defined as the total amount of energy evolved when free ions of an atom combine together to form one mole of crystal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




